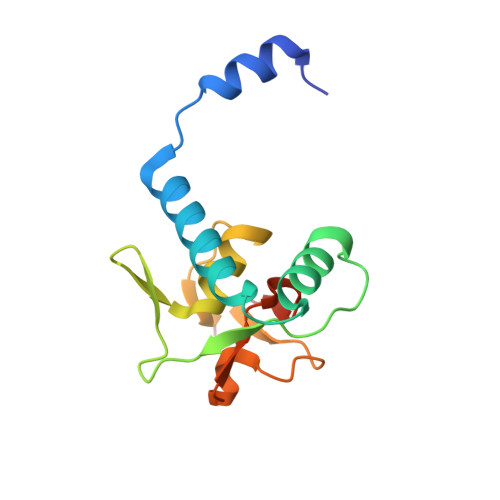
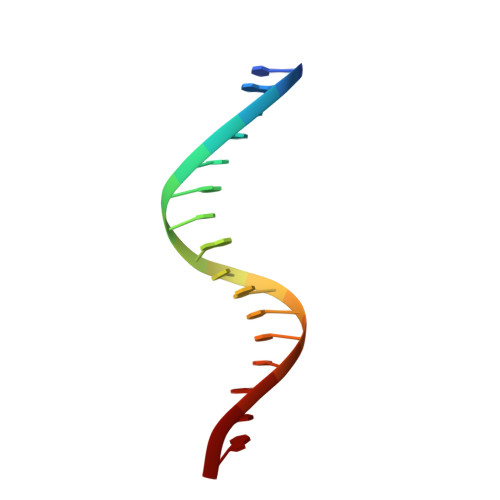
Unveiling the dimer/monomer propensities of Smad MH1-DNA complexes
Ruiz, L., Kaczmarska, Z., Gomes, T., Aragon, E., Torner, C., Freier, R., Baginski, B., Martin-Malpartida, P., Marquez, J.A., Cordeiro, T.N., Pluta, R., Macias, M.J.(2021) Computational And Structural Biotechnology Journal 19