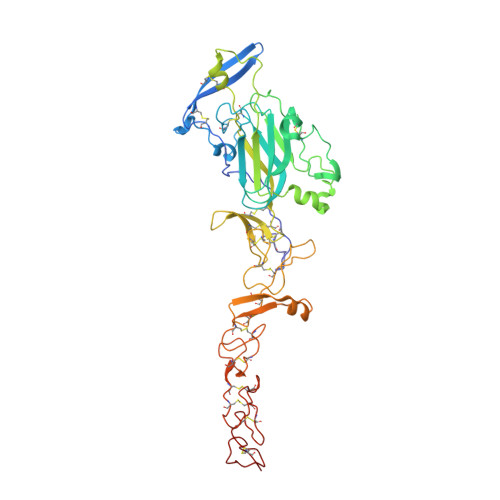
Structural Basis for Draxin-Modulated Axon Guidance and Fasciculation by Netrin-1 through DCC.
Liu, Y., Bhowmick, T., Liu, Y., Gao, X., Mertens, H.D.T., Svergun, D.I., Xiao, J., Zhang, Y., Wang, J.H., Meijers, R.(2018) Neuron 97: 1261-1267.e4
- PubMed: 29503192
- DOI: https://doi.org/10.1016/j.neuron.2018.02.010
- Primary Citation of Related Structures:
5Z5K, 6FKQ - PubMed Abstract:
Axon guidance involves the spatiotemporal interplay between guidance cues and membrane-bound cell-surface receptors, present on the growth cone of the axon. Netrin-1 is a prototypical guidance cue that binds to deleted in colorectal cancer (DCC), and it has been proposed that the guidance cue Draxin modulates this interaction. Here, we present structural snapshots of Draxin/DCC and Draxin/Netrin-1 complexes, revealing a triangular relationship that affects Netrin-mediated haptotaxis and fasciculation. Draxin interacts with DCC through the N-terminal four immunoglobulin domains, and Netrin-1 through the EGF-3 domain, in the same region where DCC binds. Netrin-1 and DCC bind to adjacent sites on Draxin, which appears to capture Netrin-1 and tether it to the DCC receptor. We propose the conformational flexibility of the single-pass membrane receptor DCC is used to promote fasciculation and regulate axon guidance through concerted Netrin-1/Draxin binding. VIDEO ABSTRACT.
- State Key Laboratory of Membrane Biology, College of Life Sciences, Peking University, Beijing 100871, China.
Organizational Affiliation: