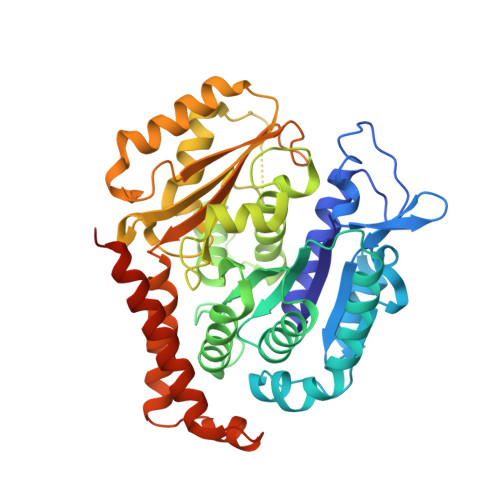
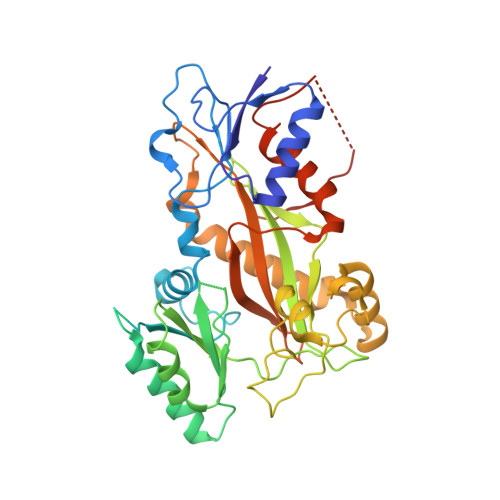
A fluorescence anisotropy assay to discover and characterize ligands targeting the maytansine site of tubulin.
Menchon, G., Prota, A.E., Lucena-Agell, D., Bucher, P., Jansen, R., Irschik, H., Muller, R., Paterson, I., Diaz, J.F., Altmann, K.H., Steinmetz, M.O.(2018) Nat Commun 9: 2106-2106
- PubMed: 29844393
- DOI: https://doi.org/10.1038/s41467-018-04535-8
- Primary Citation of Related Structures:
6FII, 6FJF, 6FJM - PubMed Abstract:
Microtubule-targeting agents (MTAs) like taxol and vinblastine are among the most successful chemotherapeutic drugs against cancer. Here, we describe a fluorescence anisotropy-based assay that specifically probes for ligands targeting the recently discovered maytansine site of tubulin. Using this assay, we have determined the dissociation constants of known maytansine site ligands, including the pharmacologically active degradation product of the clinical antibody-drug conjugate trastuzumab emtansine. In addition, we discovered that the two natural products spongistatin-1 and disorazole Z with established cellular potency bind to the maytansine site on β-tubulin. The high-resolution crystal structures of spongistatin-1 and disorazole Z in complex with tubulin allowed the definition of an additional sub-site adjacent to the pocket shared by all maytansine-site ligands, which could be exploitable as a distinct, separate target site for small molecules. Our study provides a basis for the discovery and development of next-generation MTAs for the treatment of cancer.
- Laboratory of Biomolecular Research, Division of Biology and Chemistry, Paul Scherrer Institut, Villigen PSI, 5232, Switzerland.
Organizational Affiliation: