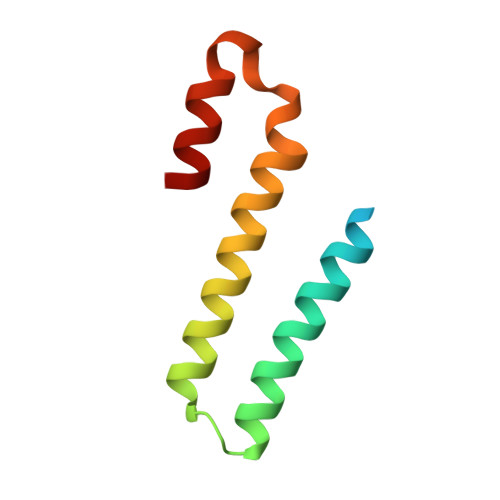
Structural plasticity of the HHD2 domain of whirlin.
Delhommel, F., Cordier, F., Saul, F., Chataigner, L., Haouz, A., Wolff, N.(2018) FEBS J 285: 3738-3752
- PubMed: 30053338
- DOI: https://doi.org/10.1111/febs.14614
- Primary Citation of Related Structures:
6FDD, 6FDE - PubMed Abstract:
Whirlin is a protein essential to sensory neurons. Its defects are responsible for nonsyndromic deafness or for the Usher syndrome, a condition associating congenital deafness and progressive blindness. This large multidomain scaffolding protein is expressed in three isoforms with different functions and localizations in stereocilia bundles of hearing hair cells or in the connecting cilia of photoreceptor cells. The HHD2 domain of whirlin is the only domain shared by all isoforms, but its function remains unknown. In this article, we report its crystal structure in two distinct conformations, a monomeric five-helix bundle, similar to the known structure of other HHD domains, and a three-helix bundle organized as a swapped dimer. Most of the hydrophobic contacts and electrostatic interactions that maintain the globular monomeric form are conserved at the protomer interface of the dimer. NMR experiments revealed that the five-helix conformation is predominant in solution, but exhibits increased dynamics on one face encompassing the hinge loops. Using NMR and SAXS, we also show that HHD2 does not interact with its preceding domains. Our findings suggest that structural plasticity might play a role in the function of the HHD2 domain.
- Unité Récepteurs-Canaux, Institut Pasteur, Paris, France.
Organizational Affiliation: