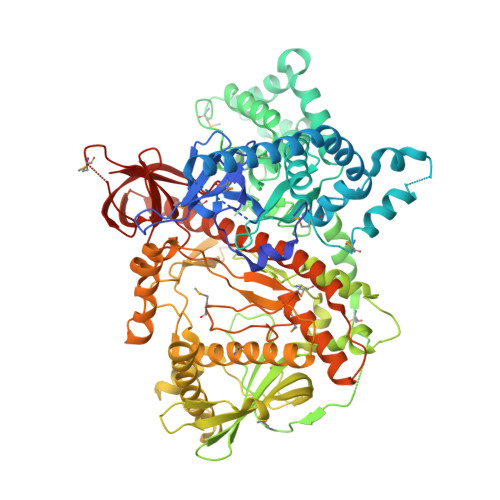
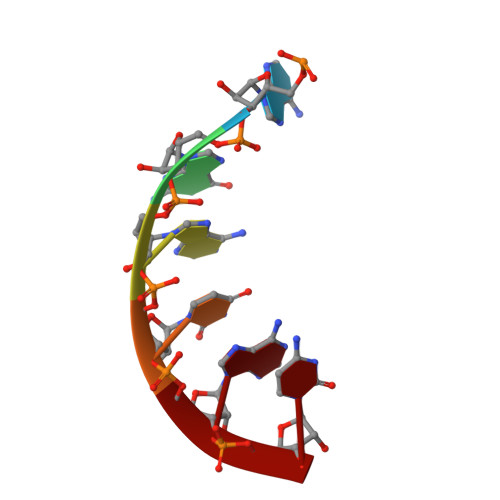
Structural analysis of mtEXO mitochondrial RNA degradosome reveals tight coupling of nuclease and helicase components.
Razew, M., Warkocki, Z., Taube, M., Kolondra, A., Czarnocki-Cieciura, M., Nowak, E., Labedzka-Dmoch, K., Kawinska, A., Piatkowski, J., Golik, P., Kozak, M., Dziembowski, A., Nowotny, M.(2018) Nat Commun 9: 97-97
- PubMed: 29311576
- DOI: https://doi.org/10.1038/s41467-017-02570-5
- Primary Citation of Related Structures:
6F3H, 6F4A - PubMed Abstract:
Nuclease and helicase activities play pivotal roles in various aspects of RNA processing and degradation. These two activities are often present in multi-subunit complexes from nucleic acid metabolism. In the mitochondrial exoribonuclease complex (mtEXO) both enzymatic activities are tightly coupled making it an excellent minimal system to study helicase-exoribonuclease coordination. mtEXO is composed of Dss1 3'-to-5' exoribonuclease and Suv3 helicase. It is the master regulator of mitochondrial gene expression in yeast. Here, we present the structure of mtEXO and a description of its mechanism of action. The crystal structure of Dss1 reveals domains that are responsible for interactions with Suv3. Importantly, these interactions are compatible with the conformational changes of Suv3 domains during the helicase cycle. We demonstrate that mtEXO is an intimate complex which forms an RNA-binding channel spanning its entire structure, with Suv3 helicase feeding the 3' end of the RNA toward the active site of Dss1.
- Laboratory of Protein Structure, International Institute of Molecular and Cell Biology, Trojdena 4, 02-109, Warsaw, Poland.
Organizational Affiliation: