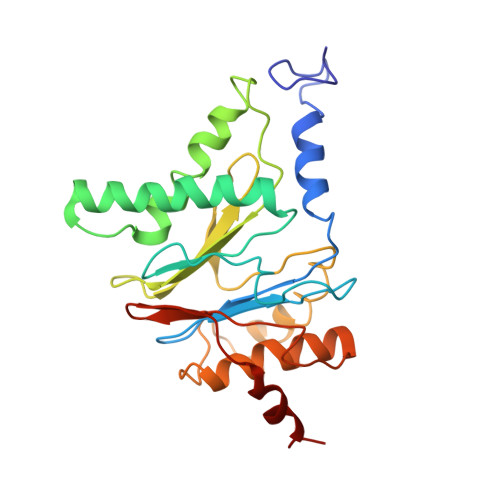
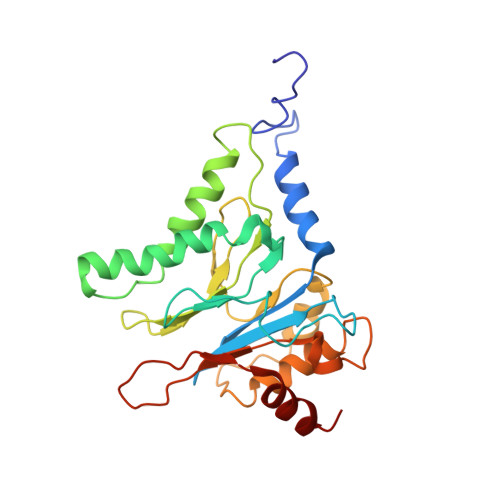
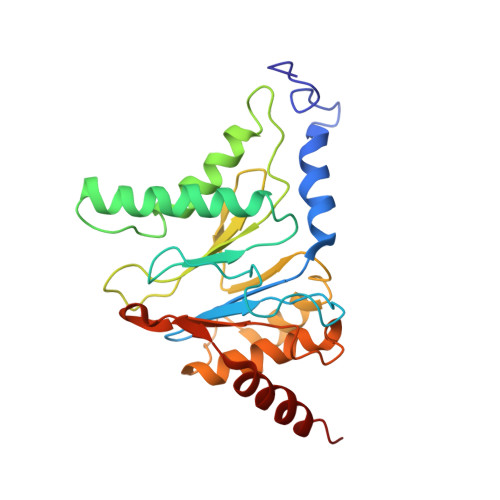
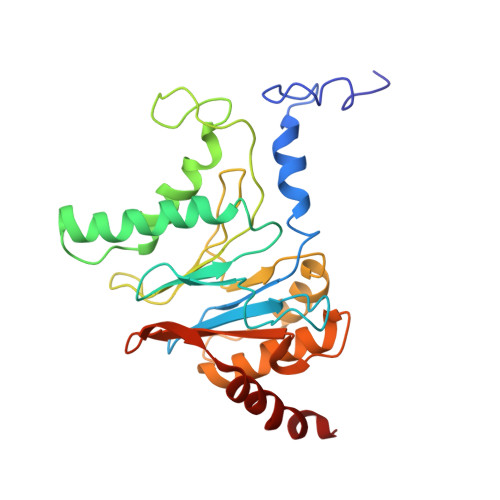
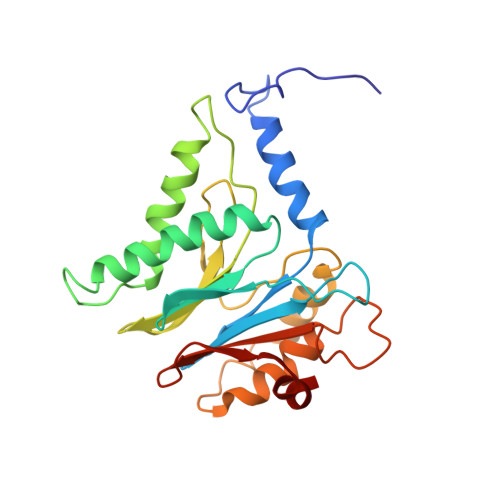
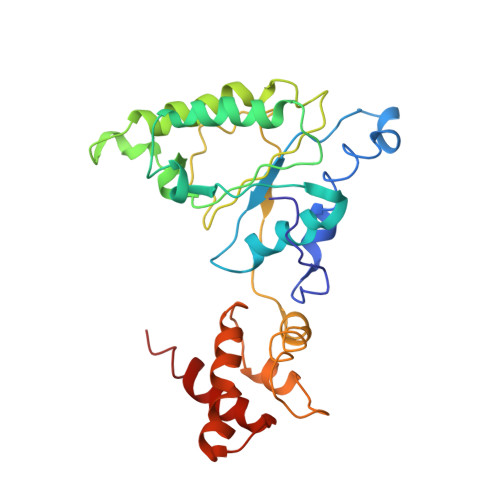
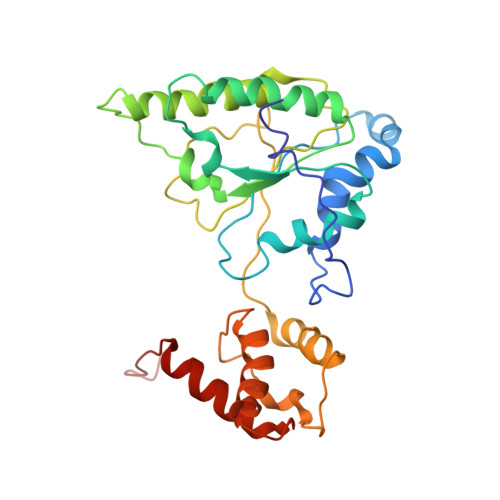
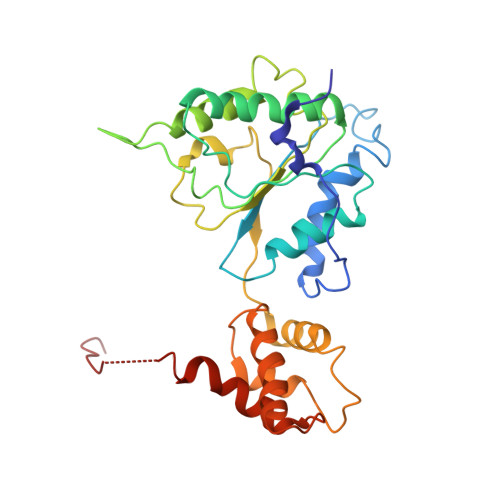
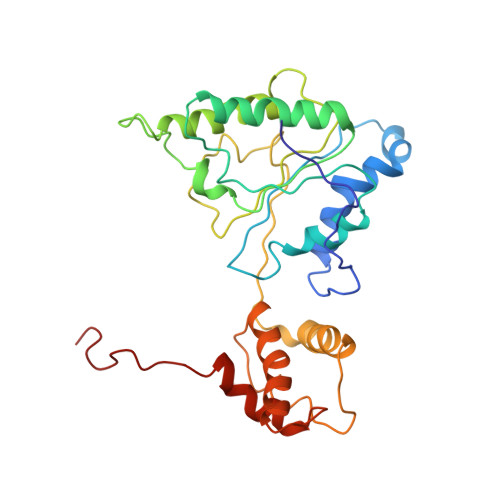
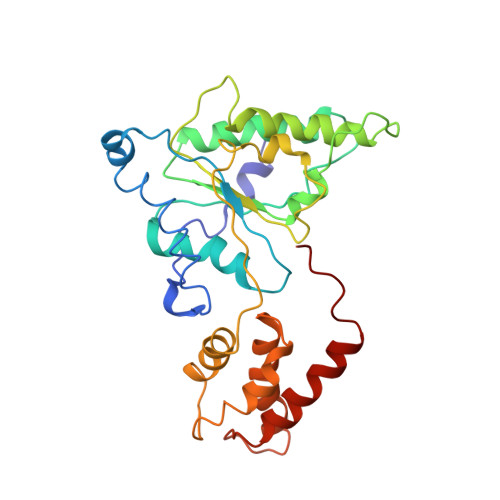
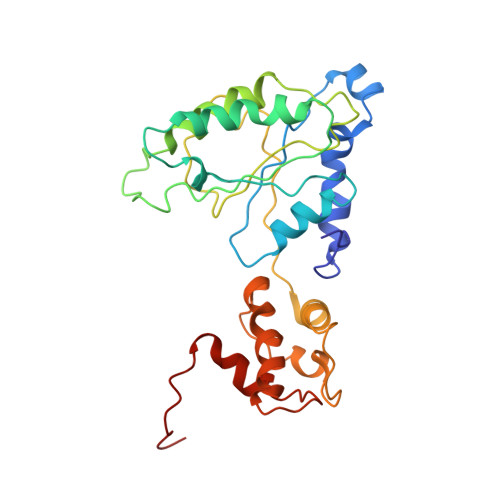
Substrate-engaged 26Sproteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation.
de la Pena, A.H., Goodall, E.A., Gates, S.N., Lander, G.C., Martin, A.(2018) Science 362
- PubMed: 30309908
- DOI: https://doi.org/10.1126/science.aav0725
- Primary Citation of Related Structures:
6EF0, 6EF1, 6EF2, 6EF3 - PubMed Abstract:
The 26 S proteasome is the primary eukaryotic degradation machine and thus is critically involved in numerous cellular processes. The heterohexameric adenosine triphosphatase (ATPase) motor of the proteasome unfolds and translocates targeted protein substrates into the open gate of a proteolytic core while a proteasomal deubiquitinase concomitantly removes substrate-attached ubiquitin chains. However, the mechanisms by which ATP hydrolysis drives the conformational changes responsible for these processes have remained elusive. Here we present the cryo-electron microscopy structures of four distinct conformational states of the actively ATP-hydrolyzing, substrate-engaged 26 S proteasome. These structures reveal how mechanical substrate translocation accelerates deubiquitination and how ATP-binding, -hydrolysis, and phosphate-release events are coordinated within the AAA+ (ATPases associated with diverse cellular activities) motor to induce conformational changes and propel the substrate through the central pore.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: