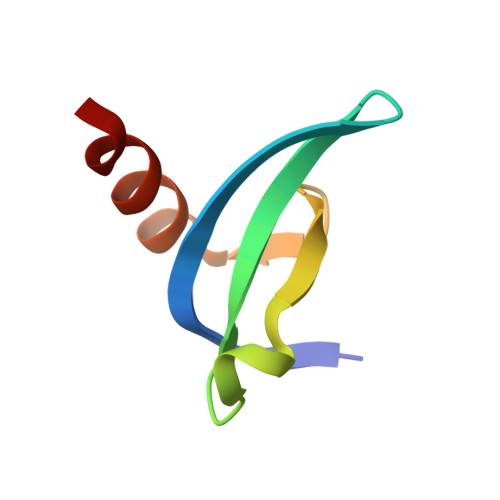
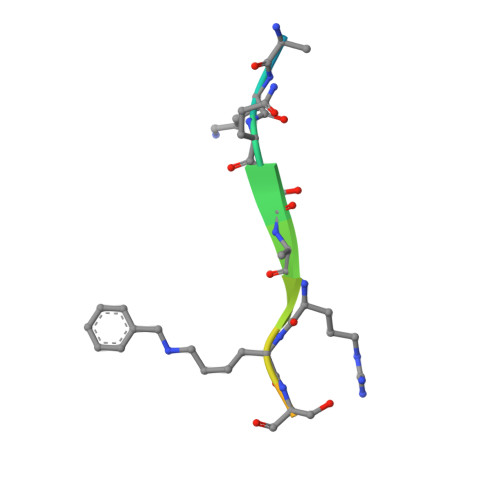
Engineering Methyllysine Writers and Readers for Allele-Specific Regulation of Protein-Protein Interactions.
Arora, S., Horne, W.S., Islam, K.(2019) J Am Chem Soc 141: 15466-15470
- PubMed: 31518125
- DOI: https://doi.org/10.1021/jacs.9b05725
- Primary Citation of Related Structures:
6D07, 6D08 - PubMed Abstract:
Protein-protein interactions mediated by methyllysine are ubiquitous in biological systems. Specific perturbation of such interactions has remained a challenging endeavor. Herein, we describe an allele-specific strategy toward an engineered protein-protein interface orthogonal to the human proteome. We develop a methyltransferase (writer) variant that installs aryllysine moiety on histones that can only be recognized by an engineered chromodomain (reader). We establish biochemical integrity of the engineered interface, provide structural evidence for orthogonality and validate its applicability to identify transcriptional regulators. Our approach provides an unprecedented strategy for specific manipulation of the methyllysine interactome.
- Department of Chemistry , University of Pittsburgh , Pittsburgh , Pennsylvania 15260 , United States.
Organizational Affiliation: