Discovery of Selective RNA-Binding Small Molecules by Affinity-Selection Mass Spectrometry.
Rizvi, N.F., Howe, J.A., Nahvi, A., Klein, D.J., Fischmann, T.O., Kim, H.Y., McCoy, M.A., Walker, S.S., Hruza, A., Richards, M.P., Chamberlin, C., Saradjian, P., Butko, M.T., Mercado, G., Burchard, J., Strickland, C., Dandliker, P.J., Smith, G.F., Nickbarg, E.B.(2018) ACS Chem Biol 13: 820-831
- PubMed: 29412640
- DOI: https://doi.org/10.1021/acschembio.7b01013
- Primary Citation of Related Structures:
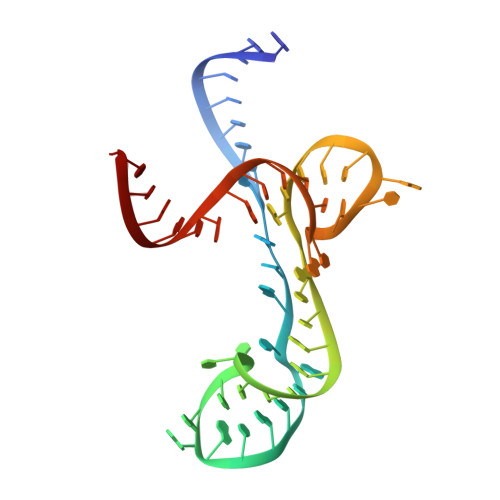
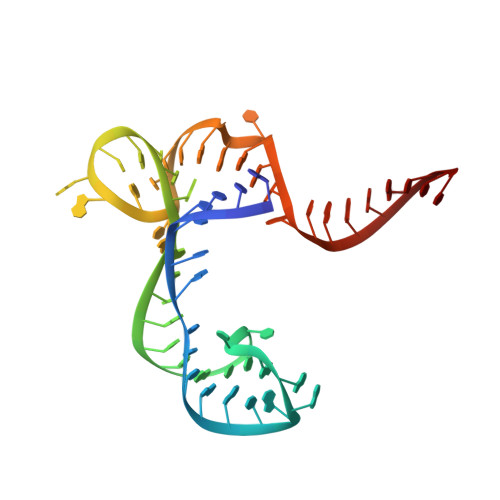
6BFB - PubMed Abstract:
Recent advances in understanding the relevance of noncoding RNA (ncRNA) to disease have increased interest in drugging ncRNA with small molecules. The recent discovery of ribocil, a structurally distinct synthetic mimic of the natural ligand of the flavin mononucleotide (FMN) riboswitch, has revealed the potential chemical diversity of small molecules that target ncRNA. Affinity-selection mass spectrometry (AS-MS) is theoretically applicable to high-throughput screening (HTS) of small molecules binding to ncRNA. Here, we report the first application of the Automated Ligand Detection System (ALIS), an indirect AS-MS technique, for the selective detection of small molecule-ncRNA interactions, high-throughput screening against large unbiased small-molecule libraries, and identification and characterization of novel compounds (structurally distinct from both FMN and ribocil) that target the FMN riboswitch. Crystal structures reveal that different compounds induce various conformations of the FMN riboswitch, leading to different activity profiles. Our findings validate the ALIS platform for HTS screening for RNA-binding small molecules and further demonstrate that ncRNA can be broadly targeted by chemically diverse yet selective small molecules as therapeutics.
- Merck & Co., Inc. , Boston , Massachusetts 02115 , United States.
Organizational Affiliation: