Structure-function characterization of three human antibodies targeting the vaccinia virus adhesion molecule D8.
Matho, M.H., Schlossman, A., Gilchuk, I.M., Miller, G., Mikulski, Z., Hupfer, M., Wang, J., Bitra, A., Meng, X., Xiang, Y., Kaever, T., Doukov, T., Ley, K., Crotty, S., Peters, B., Hsieh-Wilson, L.C., Crowe, J.E., Zajonc, D.M.(2018) J Biological Chem 293: 390-401
- PubMed: 29123031
- DOI: https://doi.org/10.1074/jbc.M117.814541
- Primary Citation of Related Structures:
5USH, 5USL, 6B9J - PubMed Abstract:
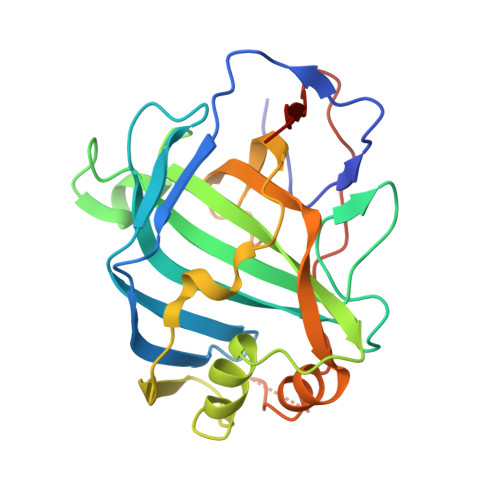
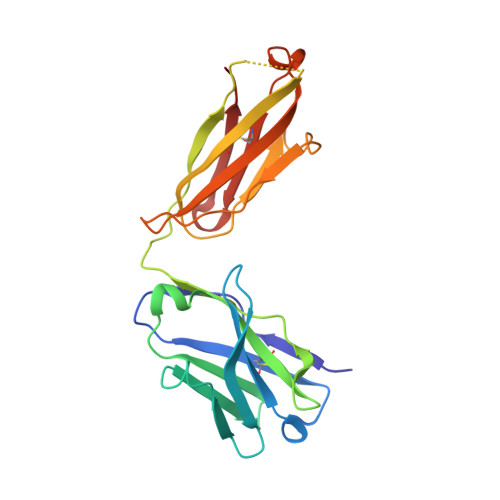
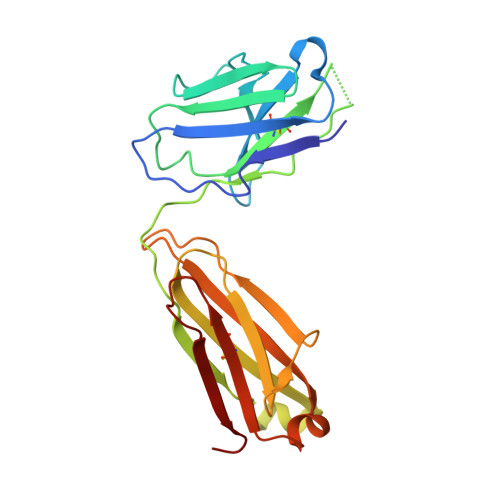
Vaccinia virus (VACV) envelope protein D8 is one of three glycosaminoglycan adhesion molecules and binds to the linear polysaccharide chondroitin sulfate (CS). D8 is also a target for neutralizing antibody responses that are elicited by the smallpox vaccine, which has enabled the first eradication of a human viral pathogen and is a useful model for studying antibody responses. However, to date, VACV epitopes targeted by human antibodies have not been characterized at atomic resolution. Here, we characterized the binding properties of several human anti-D8 antibodies and determined the crystal structures of three VACV-mAb variants, VACV-66, VACV-138, and VACV-304, separately bound to D8. Although all these antibodies bound D8 with high affinity and were moderately neutralizing in the presence of complement, VACV-138 and VACV-304 also fully blocked D8 binding to CS-A, the low affinity ligand for D8. VACV-138 also abrogated D8 binding to the high-affinity ligand CS-E, but we observed residual CS-E binding was observed in the presence of VACV-304. Analysis of the VACV-138- and VACV-304-binding sites along the CS-binding crevice of D8, combined with different efficiencies of blocking D8 adhesion to CS-A and CS-E allowed us to propose that D8 has a high- and low-affinity CS-binding region within its central crevice. The crevice is amenable to protein engineering to further enhance both specificity and affinity of binding to CS-E. Finally, a wild-type D8 tetramer specifically bound to structures within the developing glomeruli of the kidney, which express CS-E. We propose that through structure-based protein engineering, an improved D8 tetramer could be used as a potential diagnostic tool to detect expression of CS-E, which is a possible biomarker for ovarian cancer.
- Division of Cell Biology, La Jolla, California 92037.
Organizational Affiliation: