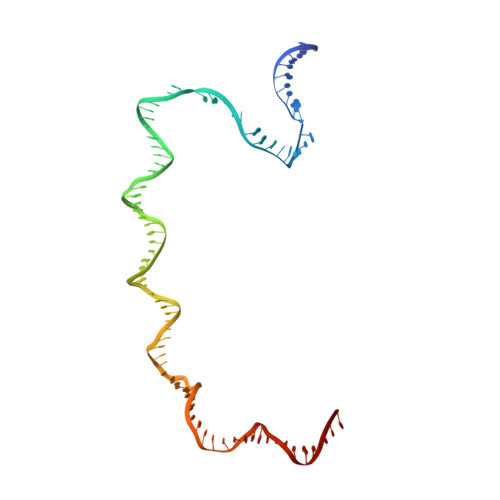
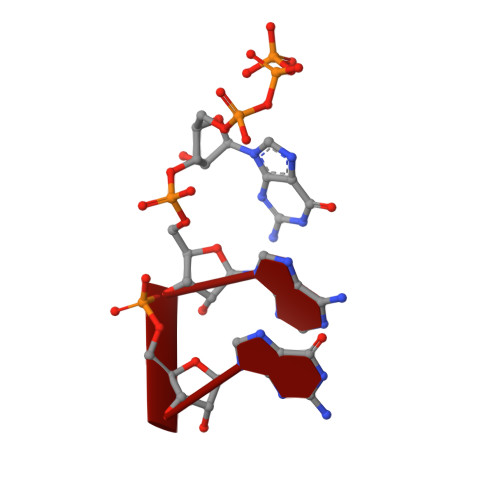
Structural basis of bacterial transcription activation.
Liu, B., Hong, C., Huang, R.K., Yu, Z., Steitz, T.A.(2017) Science 358: 947-951
- PubMed: 29146813
- DOI: https://doi.org/10.1126/science.aao1923
- Primary Citation of Related Structures:
6B6H - PubMed Abstract:
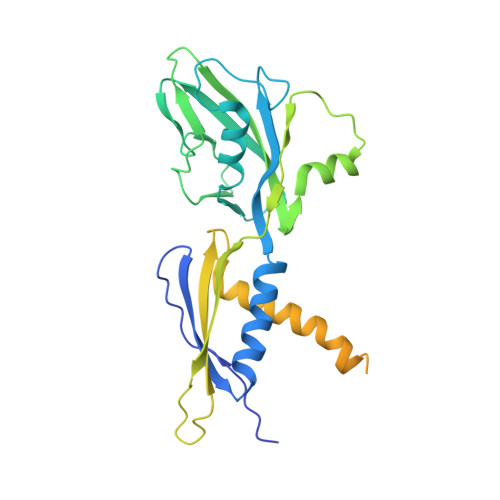
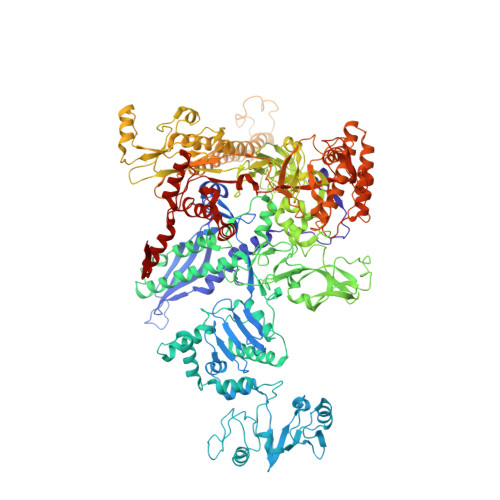
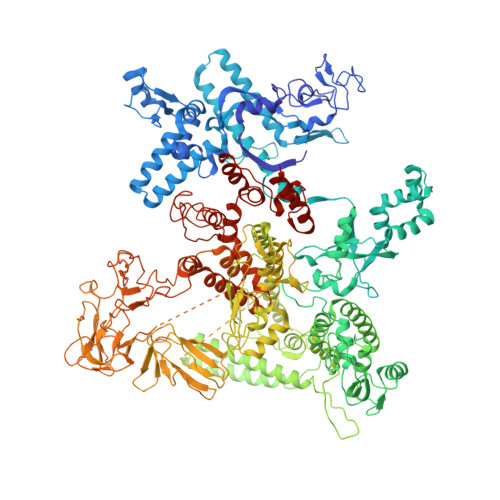
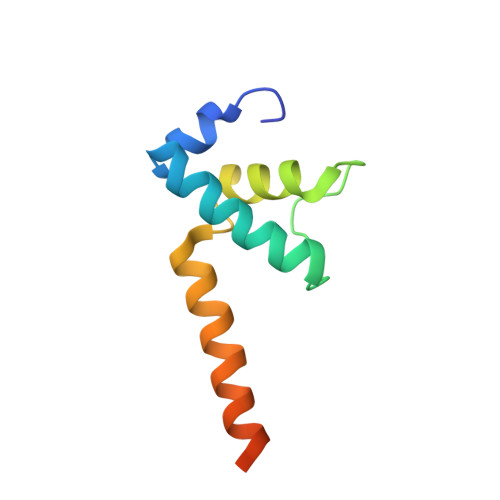
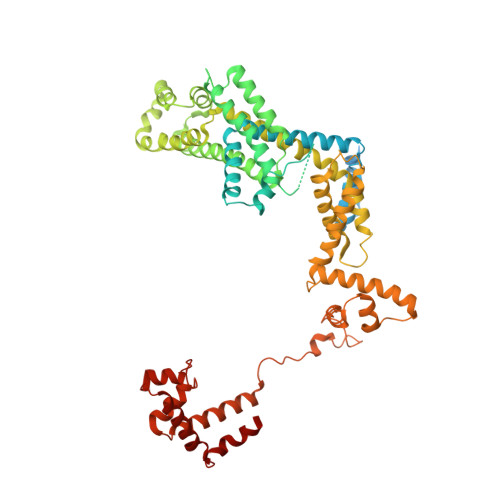
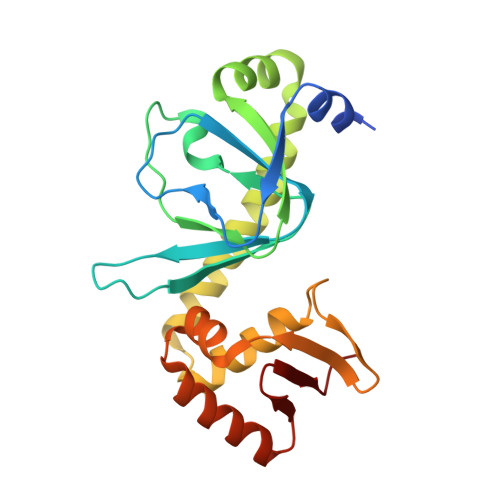
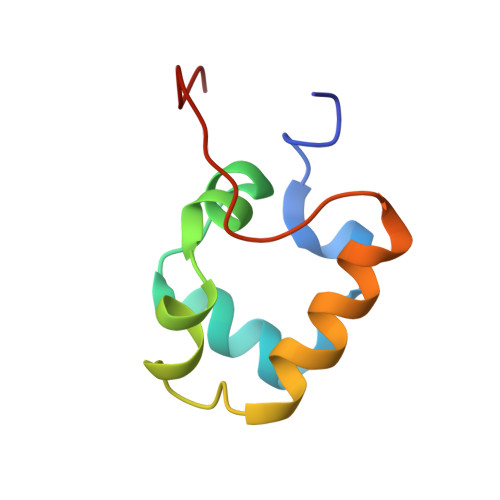
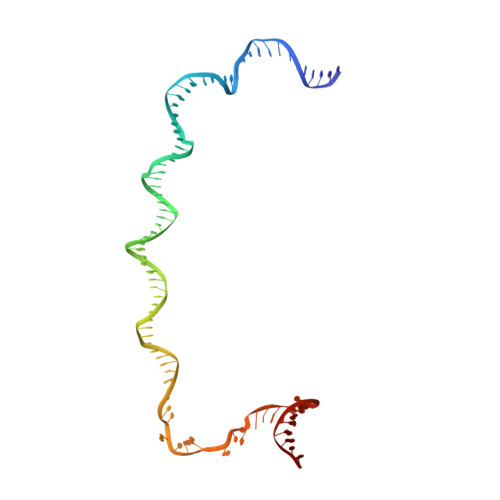
In bacteria, the activation of gene transcription at many promoters is simple and only involves a single activator. The cyclic adenosine 3',5'-monophosphate receptor protein (CAP), a classic activator, is able to activate transcription independently through two different mechanisms. Understanding the class I mechanism requires an intact transcription activation complex (TAC) structure at a high resolution. Here we report a high-resolution cryo-electron microscopy structure of an intact Escherichia coli class I TAC containing a CAP dimer, a σ 70 -RNA polymerase (RNAP) holoenzyme, a complete class I CAP-dependent promoter DNA, and a de novo synthesized RNA oligonucleotide. The structure shows how CAP wraps the upstream DNA and how the interactions recruit RNAP. Our study provides a structural basis for understanding how activators activate transcription through the class I recruitment mechanism.
- Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA.
Organizational Affiliation: