Osmolyte binding capacity of a dual action IMPase/FBPase (AF2372)
Goldstein, R.I., Roberts, M.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fructose-1,6-bisphosphatase/inositol-1-monophosphatase | 252 | Archaeoglobus fulgidus DSM 4304 | Mutation(s): 2 Gene Names: suhB, AF_2372 EC: 3.1.3.11 (PDB Primary Data), 3.1.3.25 (PDB Primary Data) | 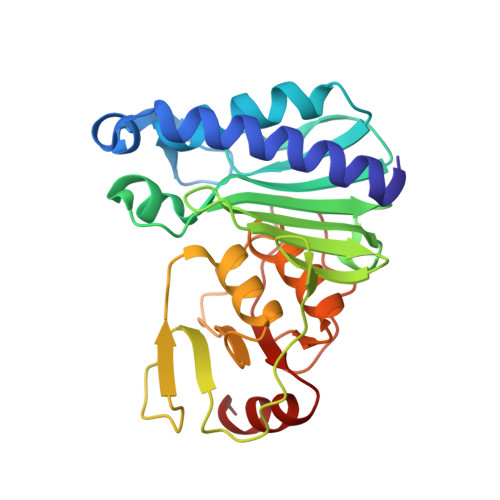 | |
UniProt | |||||
Find proteins for O30298 (Archaeoglobus fulgidus (strain ATCC 49558 / DSM 4304 / JCM 9628 / NBRC 100126 / VC-16)) Explore O30298 Go to UniProtKB: O30298 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O30298 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PGE Query on PGE | K [auth A] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| GLU (Subject of Investigation/LOI) Query on GLU | C [auth A], D [auth A], L [auth B], M [auth B] | GLUTAMIC ACID C5 H9 N O4 WHUUTDBJXJRKMK-VKHMYHEASA-N |  | ||
| SO4 Query on SO4 | H [auth A], I [auth A], J [auth A], Q [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | E [auth A], N [auth B], R [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| MG Query on MG | F [auth A], G [auth A], O [auth B], P [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.906 | α = 90 |
| b = 89.906 | β = 90 |
| c = 100.935 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Department of Energy (DOE, United States) | United States | DE-FG02-91-ER20025 |