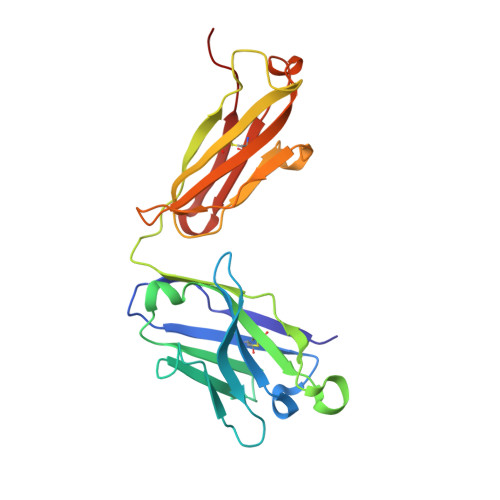
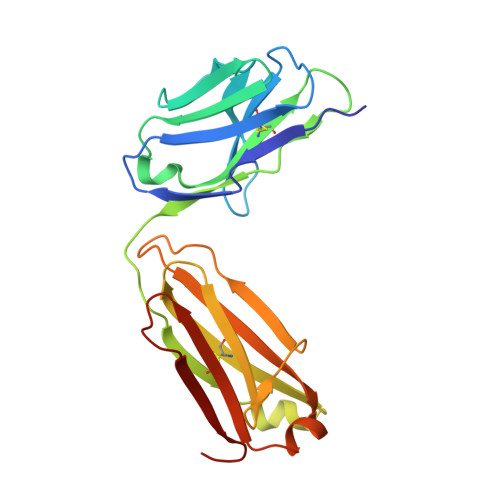
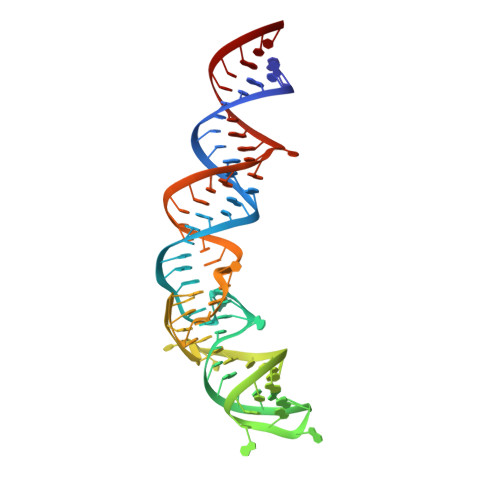
Affinity maturation of a portable Fab-RNA module for chaperone-assisted RNA crystallography.
Koirala, D., Shelke, S.A., Dupont, M., Ruiz, S., DasGupta, S., Bailey, L.J., Benner, S.A., Piccirilli, J.A.(2018) Nucleic Acids Res 46: 2624-2635
- PubMed: 29309709
- DOI: https://doi.org/10.1093/nar/gkx1292
- Primary Citation of Related Structures:
6B14, 6B3K - PubMed Abstract:
Antibody fragments such as Fabs possess properties that can enhance protein and RNA crystallization and therefore can facilitate macromolecular structure determination. In particular, Fab BL3-6 binds to an AAACA RNA pentaloop closed by a GC pair with ∼100 nM affinity. The Fab and hairpin have served as a portable module for RNA crystallization. The potential for general application make it desirable to adjust the properties of this crystallization module in a manner that facilitates its use for RNA structure determination, such as ease of purification, surface entropy or binding affinity. In this work, we used both in vitro RNA selection and phage display selection to alter the epitope and paratope sides of the binding interface, respectively, for improved binding affinity. We identified a 5'-GNGACCC-3' consensus motif in the RNA and S97N mutation in complimentarity determining region L3 of the Fab that independently impart about an order of magnitude improvement in affinity, resulting from new hydrogen bonding interactions. Using a model RNA, these modifications facilitated crystallization under a wider range of conditions and improved diffraction. The improved features of the Fab-RNA module may facilitate its use as an affinity tag for RNA purification and imaging and as a chaperone for RNA crystallography.
- Department of Biochemistry and Molecular Biology, The University of Chicago, Chicago, IL 60637, USA.
Organizational Affiliation: