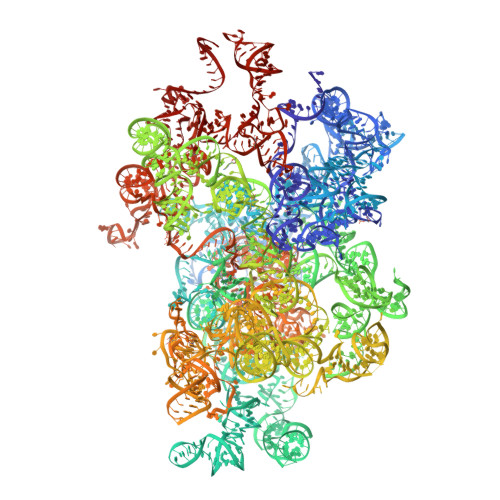
Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin.
Shalev-Benami, M., Zhang, Y., Rozenberg, H., Nobe, Y., Taoka, M., Matzov, D., Zimmerman, E., Bashan, A., Isobe, T., Jaffe, C.L., Yonath, A., Skiniotis, G.(2017) Nat Commun 8: 1589-1589
- PubMed: 29150609
- DOI: https://doi.org/10.1038/s41467-017-01664-4
- Primary Citation of Related Structures:
6AZ1, 6AZ3 - PubMed Abstract:
Leishmania is a single-celled eukaryotic parasite afflicting millions of humans worldwide, with current therapies limited to a poor selection of drugs that mostly target elements in the parasite's cell envelope. Here we determined the atomic resolution electron cryo-microscopy (cryo-EM) structure of the Leishmania ribosome in complex with paromomycin (PAR), a highly potent compound recently approved for treatment of the fatal visceral leishmaniasis (VL). The structure reveals the mechanism by which the drug induces its deleterious effects on the parasite. We further show that PAR interferes with several aspects of cytosolic translation, thus highlighting the cytosolic rather than the mitochondrial ribosome as the primary drug target. The results also highlight unique as well as conserved elements in the PAR-binding pocket that can serve as hotspots for the development of novel therapeutics.
- Faculty of Chemistry, Department of Structural Biology, Weizmann Institute of Science, Rehovot, 761001, Israel. moransb@stanford.edu.
Organizational Affiliation: