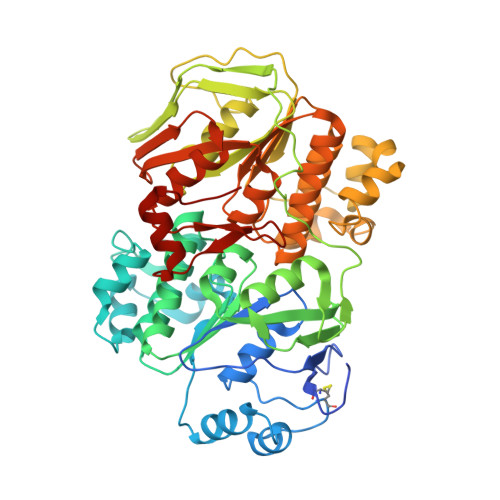
The ribosomal stalk protein is crucial for the action of the conserved ATPase ABCE1
Imai, H., Abe, T., Miyoshi, T., Nishikawa, S.I., Ito, K., Uchiumi, T.(2018) Nucleic Acids Res 46: 7820-7830
- PubMed: 30010948
- DOI: https://doi.org/10.1093/nar/gky619
- Primary Citation of Related Structures:
5YV5 - PubMed Abstract:
The ATP-binding cassette (ABC) protein ABCE1 is an essential factor in ribosome recycling during translation. However, the detailed mechanochemistry of its recruitment to the ribosome, ATPase activation and subunit dissociation remain to be elucidated. Here, we show that the ribosomal stalk protein, which is known to participate in the actions of translational GTPase factors, plays an important role in these events. Biochemical and crystal structural data indicate that the conserved hydrophobic amino acid residues at the C-terminus of the archaeal stalk protein aP1 binds to the nucleotide-binding domain 1 (NBD1) of aABCE1, and that this binding is crucial for ATPase activation of aABCE1 on the ribosome. The functional role of the stalk•ABCE1 interaction in ATPase activation and the subunit dissociation is also investigated using mutagenesis in a yeast system. The data demonstrate that the ribosomal stalk protein likely participates in efficient actions of both archaeal and eukaryotic ABCE1 in ribosome recycling. The results also show that the stalk protein has a role in the function of ATPase as well as GTPase factors in translation.
- Department of Biology, Faculty of Science, Niigata University, Ikarashi 2-8050, Nishi-ku, Niigata 950-2181, Japan.
Organizational Affiliation: