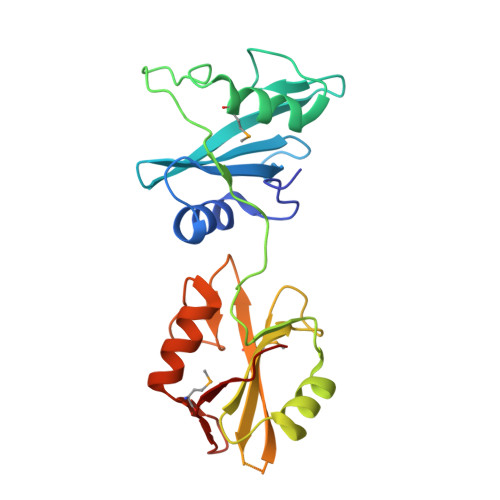
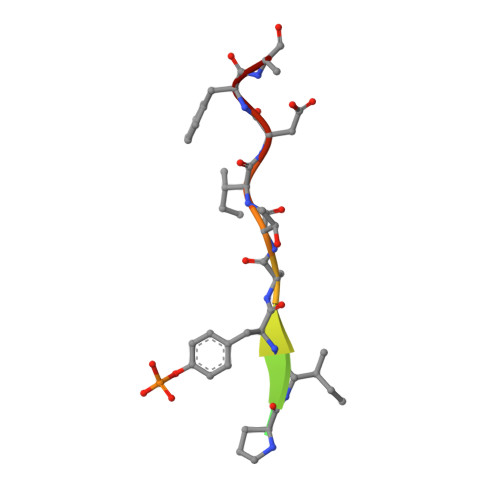
Differential Mechanisms for SHP2 Binding and Activation Are Exploited by Geographically Distinct Helicobacter pylori CagA Oncoproteins.
Hayashi, T., Senda, M., Suzuki, N., Nishikawa, H., Ben, C., Tang, C., Nagase, L., Inoue, K., Senda, T., Hatakeyama, M.(2017) Cell Rep 20: 2876-2890
- PubMed: 28930683
- DOI: https://doi.org/10.1016/j.celrep.2017.08.080
- Primary Citation of Related Structures:
5X7B, 5X94 - PubMed Abstract:
Helicobacter pylori East Asian CagA is more closely associated with gastric cancer than Western CagA. Here we show that, upon tyrosine phosphorylation, the East Asian CagA-specific EPIYA-D segment binds to the N-SH2 domain of pro-oncogenic SHP2 phosphatase two orders of magnitude greater than Western CagA-specific EPIYA-C. This high-affinity binding is achieved via cryptic interaction between Phe at the +5 position from phosphotyrosine in EPIYA-D and a hollow on the N-SH2 phosphopeptide-binding floor. Also, duplication of EPIYA-C in Western CagA, which increases gastric cancer risk, enables divalent high-affinity binding with SHP2 via N-SH2 and C-SH2. These strong CagA bindings enforce enzymatic activation of SHP2, which endows cells with neoplastic traits. Mechanistically, N-SH2 in SHP2 is in an equilibrium between stimulatory "relaxed" and inhibitory "squeezed" states, which is fixed upon high-affinity CagA binding to the "relaxed" state that stimulates SHP2. Accordingly, East Asian CagA and Western CagA exploit distinct mechanisms for SHP2 deregulation.
- Department of Microbiology, Graduate School of Medicine, The University of Tokyo, Tokyo 113-0033, Japan.
Organizational Affiliation: