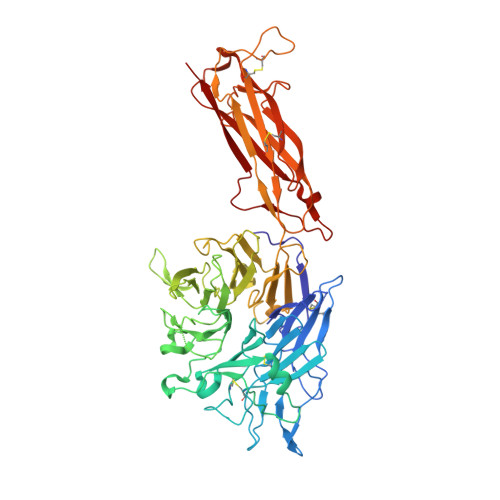
Rules of engagement between alpha v beta 6 integrin and foot-and-mouth disease virus.
Kotecha, A., Wang, Q., Dong, X., Ilca, S.L., Ondiviela, M., Zihe, R., Seago, J., Charleston, B., Fry, E.E., Abrescia, N.G.A., Springer, T.A., Huiskonen, J.T., Stuart, D.I.(2017) Nat Commun 8: 15408-15408
- PubMed: 28534487
- DOI: https://doi.org/10.1038/ncomms15408
- Primary Citation of Related Structures:
5NE4, 5NED, 5NEJ, 5NEM, 5NER, 5NET, 5NEU - PubMed Abstract:
Foot-and-mouth disease virus (FMDV) mediates cell entry by attachment to an integrin receptor, generally αvβ6, via a conserved arginine-glycine-aspartic acid (RGD) motif in the exposed, antigenic, GH loop of capsid protein VP1. Infection can also occur in tissue culture adapted virus in the absence of integrin via acquired basic mutations interacting with heparin sulphate (HS); this virus is attenuated in natural infections. HS interaction has been visualized at a conserved site in two serotypes suggesting a propensity for sulfated-sugar binding. Here we determined the interaction between αvβ6 and two tissue culture adapted FMDV strains by cryo-electron microscopy. In the preferred mode of engagement, the fully open form of the integrin, hitherto unseen at high resolution, attaches to an extended GH loop via interactions with the RGD motif plus downstream hydrophobic residues. In addition, an N-linked sugar of the integrin attaches to the previously identified HS binding site, suggesting a functional role.
- Division of Structural Biology, The Nuffield Department of Medicine, University of Oxford, The Henry Wellcome Building for Genomic Medicine, Headington, Oxford OX3 7BN, UK.
Organizational Affiliation: