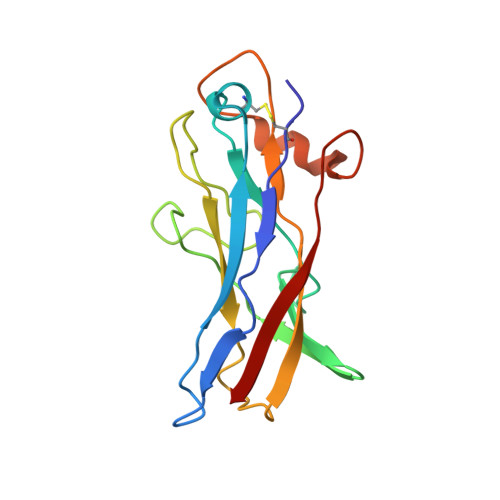
Structural and functional studies of Escherichia coli aggregative adherence fimbriae (AAF/V) reveal a deficiency in extracellular matrix binding.
Jnsson, R., Liu, B., Struve, C., Yang, Y., Jrgensen, R., Xu, Y., Jenssen, H., Krogfelt, K.A., Matthews, S.(2017) Biochim Biophys Acta 1865: 304-311
- PubMed: 27939608
- DOI: https://doi.org/10.1016/j.bbapap.2016.11.017
- Primary Citation of Related Structures:
5LVY - PubMed Abstract:
Enteroaggregative Escherichia coli (EAEC) is an emerging cause of acute and persistent diarrhea worldwide. The pathogenesis of different EAEC stains is complicated, however, the early essential step begins with attachment of EAEC to intestinal mucosa via aggregative adherence fimbriae (AAFs). Currently, five different variants have been identified, which all share a degree of similarity in the gene organization of their operons and sequences. Here, we report the solution structure of Agg5A from the AAF/V variant. While preserving the major structural features shared by all AAF members, only Agg5A possesses an inserted helix at the beginning of the donor strand, which together with altered surface electrostatics, renders the protein unable to interact with fibronectin. Hence, here we characterize the first AAF variant with a binding mode that varies from previously described AAFs.
- Institute for Science and Environment, Roskilde University, Roskilde, Denmark; Department of Microbiology and Infection Control, Statens Serum Institut, Copenhagen, Denmark.
Organizational Affiliation: