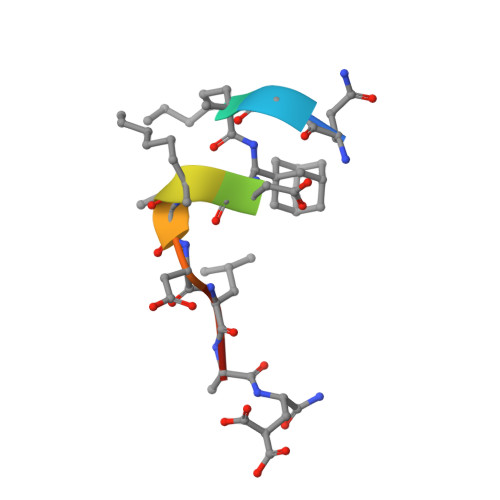
Structure-Based Design of Non-natural Macrocyclic Peptides That Inhibit Protein-Protein Interactions.
Kruger, D.M., Glas, A., Bier, D., Pospiech, N., Wallraven, K., Dietrich, L., Ottmann, C., Koch, O., Hennig, S., Grossmann, T.N.(2017) J Med Chem 60: 8982-8988
- PubMed: 29028171
- DOI: https://doi.org/10.1021/acs.jmedchem.7b01221
- Primary Citation of Related Structures:
5JM4 - PubMed Abstract:
Macrocyclic peptides can interfere with challenging biomolecular targets including protein-protein interactions. Whereas there are various approaches that facilitate the identification of peptide-derived ligands, their evolution into higher affinity binders remains a major hurdle. We report a virtual screen based on molecular docking that allows the affinity maturation of macrocyclic peptides taking non-natural amino acids into consideration. These macrocycles bear large and flexible substituents that usually complicate the use of docking approaches. A virtual library containing more than 1400 structures was screened against the target focusing on docking poses with the core structure resembling a known bioactive conformation. Based on this screen, a macrocyclic peptide 22 involving two non-natural amino acids was evolved showing increased target affinity and biological activity. Predicted binding modes were verified by X-ray crystallography. The presented workflow allows the screening of large macrocyclic peptides with diverse modifications thereby expanding the accessible chemical space and reducing synthetic efforts.
- Chemical Genomics Centre of the Max Planck Society , Otto-Hahn-Str. 15, 44227 Dortmund, Germany.
Organizational Affiliation: