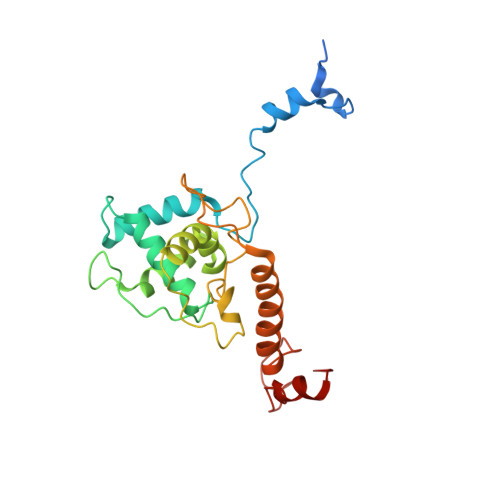
The near-atomic cryoEM structure of a flexible filamentous plant virus shows homology of its coat protein with nucleoproteins of animal viruses.
Agirrezabala, X., Mendez-Lopez, E., Lasso, G., Sanchez-Pina, M.A., Aranda, M., Valle, M.(2015) Elife 4: e11795-e11795
- PubMed: 26673077
- DOI: https://doi.org/10.7554/eLife.11795
- Primary Citation of Related Structures:
5FN1 - PubMed Abstract:
Flexible filamentous viruses include economically important plant pathogens. Their viral particles contain several hundred copies of a helically arrayed coat protein (CP) protecting a (+)ssRNA. We describe here a structure at 3.9 Å resolution, from electron cryomicroscopy, of Pepino mosaic virus (PepMV), a representative of the genus Potexvirus (family Alphaflexiviridae). Our results allow modeling of the CP and its interactions with viral RNA. The overall fold of PepMV CP resembles that of nucleoproteins (NPs) from the genus Phlebovirus (family Bunyaviridae), a group of enveloped (-)ssRNA viruses. The main difference between potexvirus CP and phlebovirus NP is in their C-terminal extensions, which appear to determine the characteristics of the distinct multimeric assemblies - a flexuous, helical rod or a loose ribonucleoprotein. The homology suggests gene transfer between eukaryotic (+) and (-)ssRNA viruses.
- Structural Biology Unit, Center for Cooperative Research in Biosciences, Derio, Spain.
Organizational Affiliation: