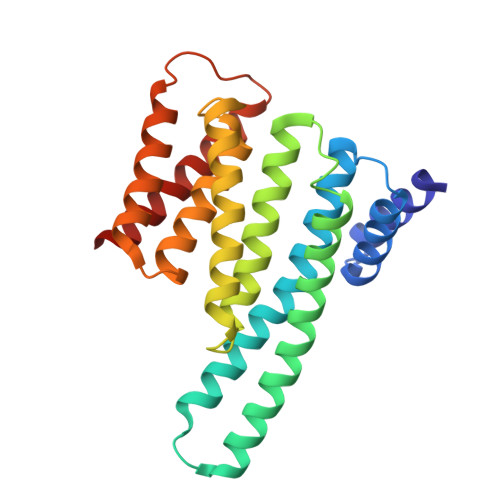
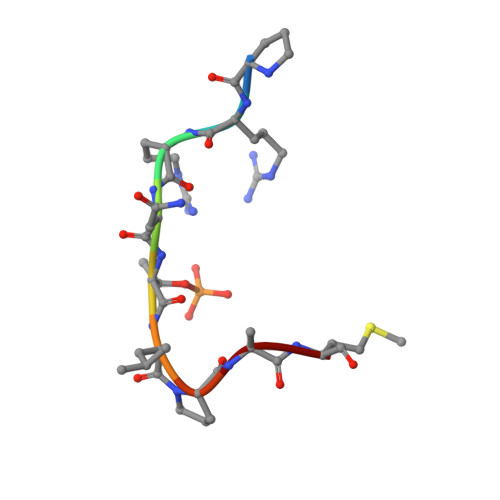
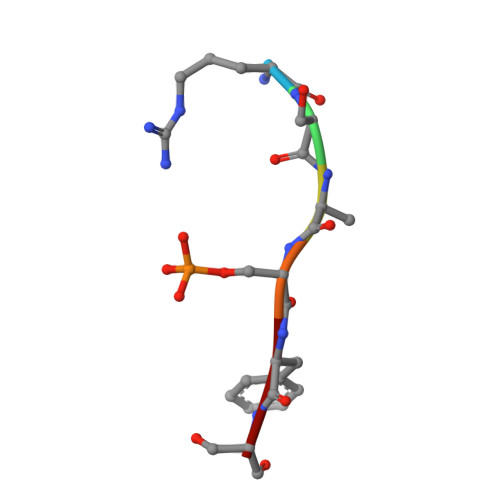
Small-Molecule Stabilization of the 14-3-3/Gab2 Protein-Protein Interaction (PPI) Interface.
Bier, D., Bartel, M., Sies, K., Halbach, S., Higuchi, Y., Haranosono, Y., Brummer, T., Kato, N., Ottmann, C.(2016) ChemMedChem 11: 911-918
- PubMed: 26644359
- DOI: https://doi.org/10.1002/cmdc.201500484
- Primary Citation of Related Structures:
5EWZ, 5EXA - PubMed Abstract:
Small-molecule modulation of protein-protein interactions (PPIs) is one of the most promising new areas in drug discovery. In the vast majority of cases only inhibition or disruption of PPIs is realized, whereas the complementary strategy of targeted stabilization of PPIs is clearly under-represented. Here, we report the example of a semi-synthetic natural product derivative--ISIR-005--that stabilizes the cancer-relevant interaction of the adaptor protein 14-3-3 and Gab2. The crystal structure of ISIR-005 in complex with 14-3-3 and the binding motif of Gab2 comprising two phosphorylation sites (Gab2pS210pT391) showed how the stabilizing molecule binds to the rim-of-the-interface of the protein complex. Only in the direct vicinity of 14-3-3/Gab2pT391 site is a pre-formed pocket occupied by ISIR-005; binding of the Gab2pS210 motif to 14-3-3 does not create an interface pocket suitable for the molecule. Accordingly, ISIR-005 only stabilizes the binding of the Gab2pT391 but not the Gab2pS210 site. This study represents structural and biochemical proof of the druggability of the 14-3-3/Gab2 PPI interface with important implications for the development of PPI stabilizers.
- Department of Chemistry, University of Duisburg-Essen, Universitätstr. 7, 45141, Essen, Germany.
Organizational Affiliation: