Solvent conditions control enzyme dynamics and catalysis
Borrguero, J.M., Cuneo, M.J., Agarwal, P.K.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
A newer entry is available that reflects an alternative modeling of the original data: 5UJX
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dihydrofolate reductase | A [auth B], B [auth A] | 159 | Escherichia coli | Mutation(s): 0 Gene Names: folA, AA953_06220, ABE89_23390, ABE90_01590, ABE91_11465, ABE92_27580, ABE93_03540, ABE94_26505, ABE95_19105, AC789_1c00510... EC: 1.5.1.3 | 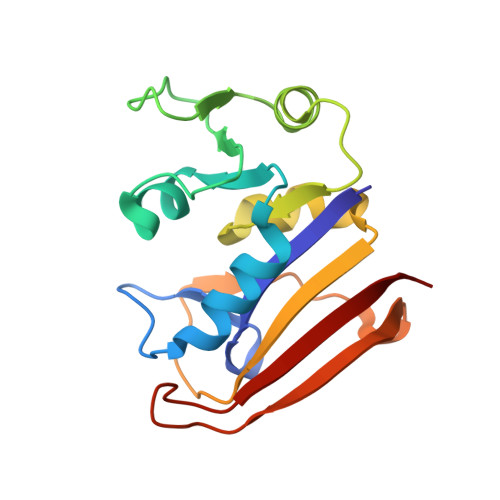 |
UniProt | |||||
Find proteins for P0ABQ4 (Escherichia coli (strain K12)) Explore P0ABQ4 Go to UniProtKB: P0ABQ4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0ABQ4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FOL Query on FOL | C [auth B], I [auth A] | FOLIC ACID C19 H19 N7 O6 OVBPIULPVIDEAO-LBPRGKRZSA-N |  | ||
| IPA Query on IPA | D [auth B] | ISOPROPYL ALCOHOL C3 H8 O KFZMGEQAYNKOFK-UHFFFAOYSA-N |  | ||
| CA Query on CA | E [auth B] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| CL Query on CL | F [auth B], G [auth B], H [auth B], J [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 91.946 | α = 90 |
| b = 91.946 | β = 90 |
| c = 73.088 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| PHASER | phasing |