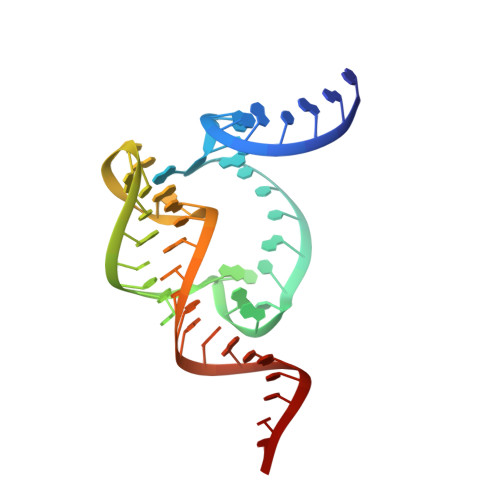
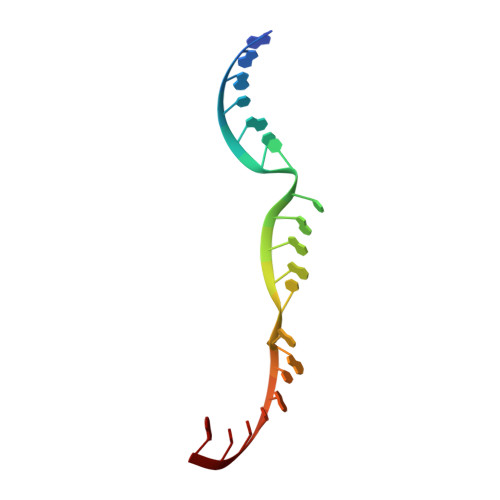
Two Divalent Metal Ions and Conformational Changes Play Roles in the Hammerhead Ribozyme Cleavage Reaction.
Mir, A., Chen, J., Robinson, K., Lendy, E., Goodman, J., Neau, D., Golden, B.L.(2015) Biochemistry 54: 6369-6381
- PubMed: 26398724
- DOI: https://doi.org/10.1021/acs.biochem.5b00824
- Primary Citation of Related Structures:
5DH6, 5DH7, 5DH8, 5DI2, 5DI4, 5DQK - PubMed Abstract:
The hammerhead ribozyme is a self-cleaving RNA broadly dispersed across all kingdoms of life. Although it was the first of the small, nucleolytic ribozymes discovered, the mechanism by which it catalyzes its reaction remains elusive. The nucleobase of G12 is well positioned to be a general base, but it is unclear if or how this guanine base becomes activated for proton transfer. Metal ions have been implicated in the chemical mechanism, but no interactions between divalent metal ions and the cleavage site have been observed crystallographically. To better understand how this ribozyme functions, we have solved crystal structures of wild-type and G12A mutant ribozymes. We observe a pH-dependent conformational change centered around G12, consistent with this nucleotide becoming deprotonated. Crystallographic and kinetic analysis of the G12A mutant reveals a Zn(2+) specificity switch suggesting a direct interaction between a divalent metal ion and the purine at position 12. The metal ion specificity switch and the pH-rate profile of the G12A mutant suggest that the minor imino tautomer of A12 serves as the general base in the mutant ribozyme. We propose a model in which the hammerhead ribozyme rearranges prior to the cleavage reaction, positioning two divalent metal ions in the process. The first metal ion, positioned near G12, becomes directly coordinated to the O6 keto oxygen, to lower the pKa of the general base and organize the active site. The second metal ion, positioned near G10.1, bridges the N7 of G10.1 and the scissile phosphate and may participate directly in the cleavage reaction.
- Department of Biochemistry, Purdue University , West Lafayette, Indiana 47907, United States.
Organizational Affiliation: