Discovery of DS-6930, a potent selective PPAR gamma modulator. Part I: Lead identification.
Shinozuka, T., Tsukada, T., Fujii, K., Tokumaru, E., Shimada, K., Onishi, Y., Matsui, Y., Wakimoto, S., Kuroha, M., Ogata, T., Araki, K., Ohsumi, J., Sawamura, R., Watanabe, N., Yamamoto, H., Fujimoto, K., Tani, Y., Mori, M., Tanaka, J.(2018) Bioorg Med Chem 26: 5079-5098
- PubMed: 30241907
- DOI: https://doi.org/10.1016/j.bmc.2018.09.006
- Primary Citation of Related Structures:
5Z5S - PubMed Abstract:
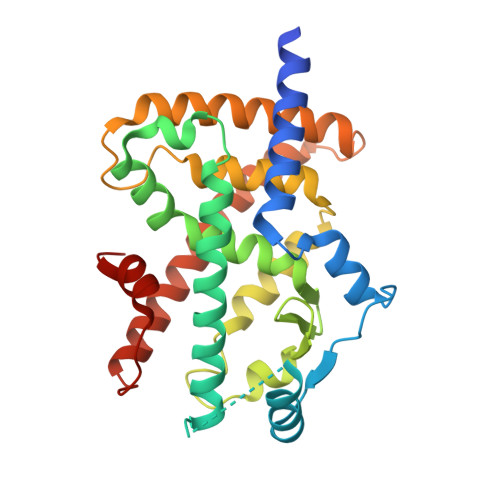
The lead identification of a novel potent selective PPARγ agonist, DS-6930 is reported. To avoid PPARγ-related adverse effects, a partial agonist was designed to prevent the direct interaction with helix 12 of PPARγ-LBD. Because the TZD group is known to interact with helix 12, the TZD in efatutazone (CS-7017) was replaced to discover novel PPARγ intermediate partial agonist 8i. The optimization of 8i yielded 13ac with high potency in vitro. Compound 13ac exhibited robust plasma glucose lowering effects comparable to those of rosiglitazone (3 mg/kg) in Zucker diabetic fatty rats. Upon toxicological evaluation, compound 13ac (300 mg/kg) induced hemodilution to a lower extent than rosiglitazone; however, 13ac elevated liver enzyme activities. X-ray crystallography revealed no direct interaction of 13ac with helix 12, and the additional lipophilic interactions are also suggested to be related to the maximum transcriptional activity of 13ac.
- R&D Division, Daiichi Sankyo Co., Ltd., 1-2-58 Hiromachi, Shinagawa-ku, Tokyo 140-8710, Japan. Electronic address: shinozuka.tsuyoshi.s5@daiichisankyo.co.jp.
Organizational Affiliation: