Structure of Glaciozyma antarctica Arginase Reveals Cold-active Adaptation via Increased Flexible Access to the Active Site
Quay, D.H.X., Yusof, N.Y., Illias, R.M., Abu-Bakar, F.D., Mahadi, N.M., Firdaus-Raih, M., Murad, A.M.A.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Arginase | 362 | Glaciozyma antarctica | Mutation(s): 0 EC: 3.5.3.1 | 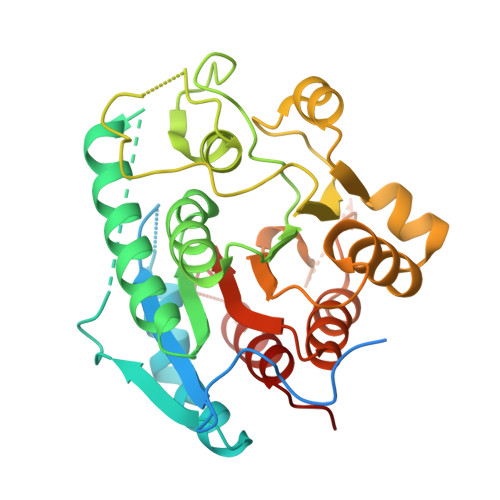 | |
UniProt | |||||
Find proteins for A0A2R2JFW7 (Glaciozyma antarctica (strain PI12)) Explore A0A2R2JFW7 Go to UniProtKB: A0A2R2JFW7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A2R2JFW7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 91.193 | α = 90 |
| b = 91.193 | β = 90 |
| c = 107.779 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| MrBUMP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministry of Science, Technology and Innovation (MOSTI, Malaysia) | Malaysia | 02-05-20-SF0007 |