Molecular mechanism of directional CTCF recognition of a diverse range of genomic sites
Yin, M., Wang, J., Wang, M., Li, X., Zhang, M., Wu, Q., Wang, Y.(2017) Cell Res 27: 1365-1377
- PubMed: 29076501
- DOI: https://doi.org/10.1038/cr.2017.131
- Primary Citation of Related Structures:
5YEF, 5YEG, 5YEH, 5YEL - PubMed Abstract:
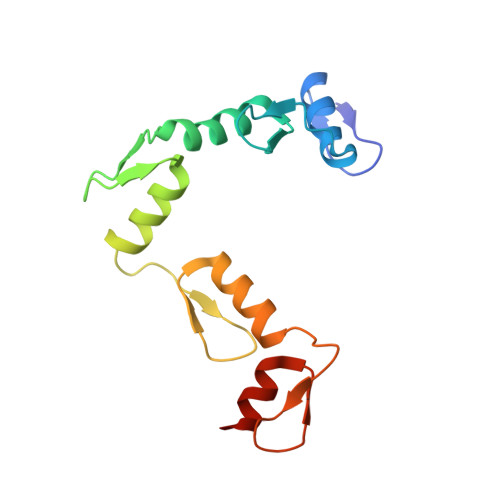
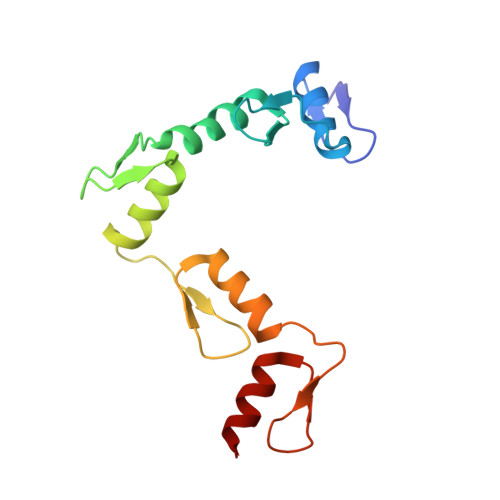
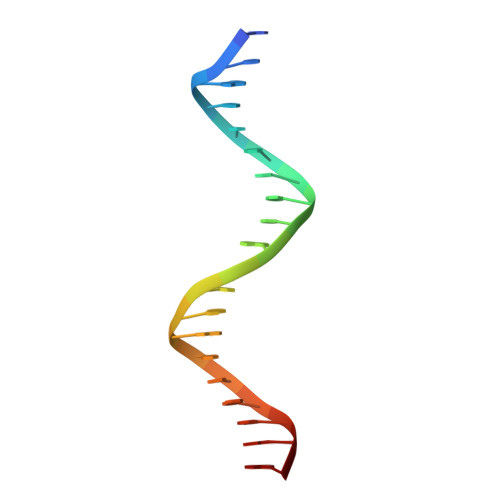
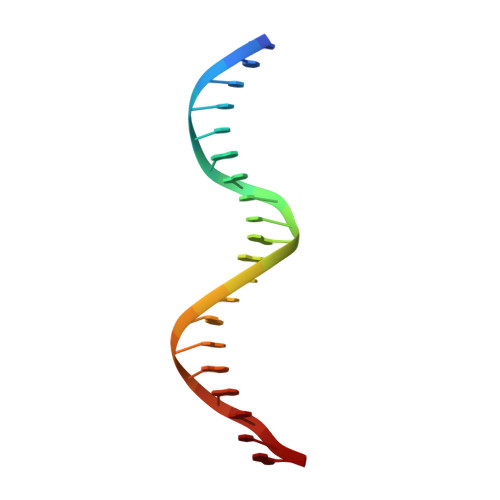
CTCF, a conserved 3D genome architecture protein, determines proper genome-wide chromatin looping interactions through directional binding to specific sequence elements of four modules within numerous CTCF-binding sites (CBSs) by its 11 zinc fingers (ZFs). Here, we report four crystal structures of human CTCF in complex with CBSs of the protocadherin (Pcdh) clusters. We show that directional CTCF binding to cognate CBSs of the Pcdh enhancers and promoters is achieved through inserting its ZF3, ZFs 4-7, and ZFs 9-11 into the major groove along CBSs, resulting in a sequence-specific recognition of module 4, modules 3 and 2, and module 1, respectively; and ZF8 serves as a spacer element for variable distances between modules 1 and 2. In addition, the base contact with the asymmetric "A" in the central position of modules 2-3, is essential for directional recognition of the CBSs with symmetric core sequences but lacking module 1. Furthermore, CTCF tolerates base changes at specific positions within the degenerated CBS sequences, permitting genome-wide CTCF binding to a diverse range of CBSs. Together, these complex structures provide important insights into the molecular mechanisms for the directionality, diversity, flexibility, dynamics, and conservation of multivalent CTCF binding to its cognate sites across the entire human genome.
- Key Laboratory of RNA Biology, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Organizational Affiliation: