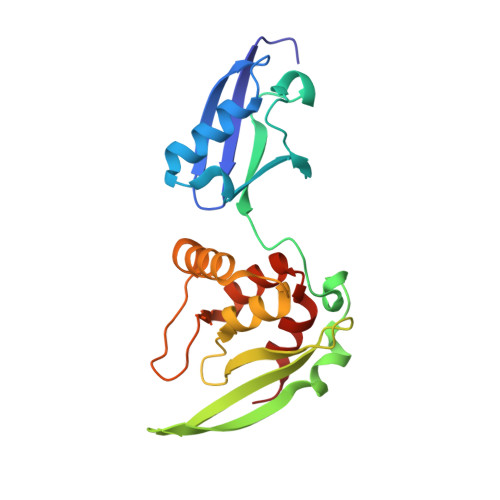
Structural insights into chromosome attachment to the nuclear envelope by an inner nuclear membrane protein Bqt4 in fission yeast.
Hu, C., Inoue, H., Sun, W., Takeshita, Y., Huang, Y., Xu, Y., Kanoh, J., Chen, Y.(2019) Nucleic Acids Res 47: 1573-1584
- PubMed: 30462301
- DOI: https://doi.org/10.1093/nar/gky1186
- Primary Citation of Related Structures:
5YC2, 5YCA, 6A6W - PubMed Abstract:
The dynamic association of chromosomes with the nuclear envelope (NE) is essential for chromosome maintenance. Schizosaccharomyces pombe inner nuclear membrane protein Bqt4 plays a critical role in connecting telomeres to the NE, mainly through a direct interaction with the telomeric protein Rap1. Bqt4 also interacts with Lem2 for pericentric heterochromatin maintenance. How Bqt4 coordinates the interactions with different proteins to exert their functions is unclear. Here, we report the crystal structures of the N-terminal domain of Bqt4 in complexes with Bqt4-binding motifs from Rap1, Lem2, and Sad1. The structural, biochemical and cellular analyses reveal that the N-terminal domain of Bqt4 is a protein-interaction module that recognizes a consensus motif and plays essential roles in telomere-NE association and meiosis progression. Phosphorylation of Bqt4-interacting proteins may act as a switch to regulate these interactions during cell cycles. Our studies provide structural insights into the identification and regulation of Bqt4-mediated interactions.
- State Key Laboratory of Molecular Biology, National Center for Protein Science Shanghai, Shanghai Science Research Center, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences; University of Chinese Academy of Sciences, 333 Haike Road, Shanghai 201210, China.
Organizational Affiliation: