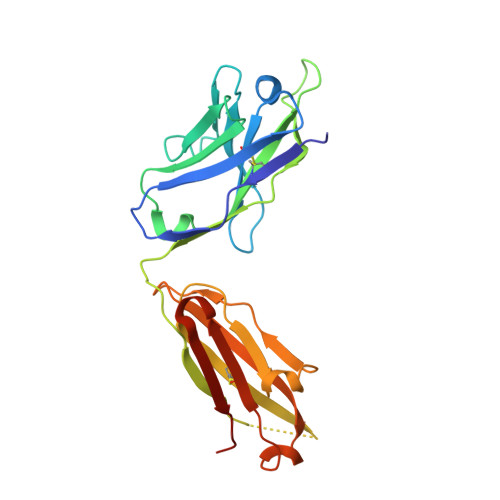
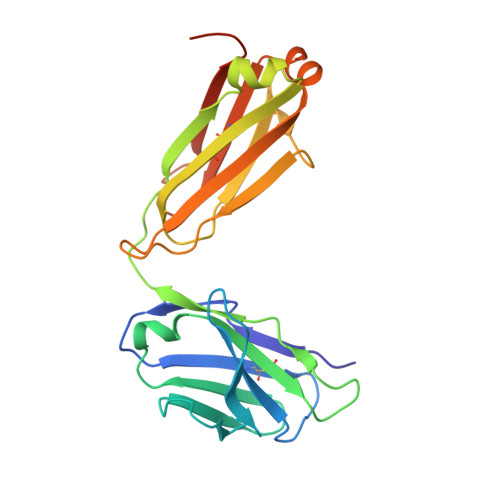
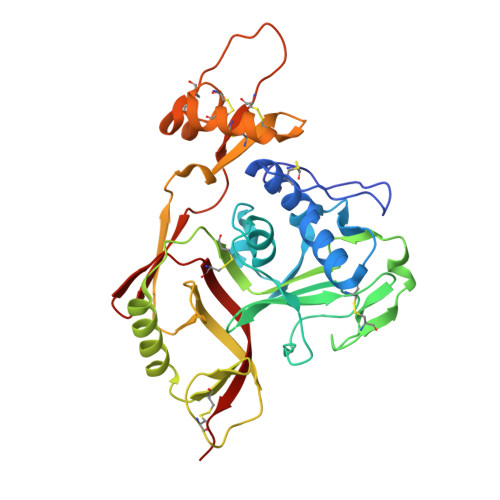
Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope
Wu, Y., Zhu, Y.H., Gao, F., Jiao, Y.J., Oladejo, B.O., Chai, Y., Bi, Y.H., Lu, S., Dong, M.Q., Zhang, C., Huang, G.M., Wong, G., Li, N., Zhang, Y.F., Li, Y., Feng, W.H., Shi, Y., Liang, M.F., Zhang, R.G., Qi, J.X., Gao, G.F.(2017) Proc Natl Acad Sci U S A 114: E7564-E7573
- PubMed: 28827346
- DOI: https://doi.org/10.1073/pnas.1705176114
- Primary Citation of Related Structures:
5Y0W, 5Y0Y, 5Y10, 5Y11 - PubMed Abstract:
Severe fever with thrombocytopenia syndrome virus (SFTSV) and Rift Valley fever virus (RVFV) are two arthropod-borne phleboviruses in the Bunyaviridae family, which cause severe illness in humans and animals. Glycoprotein N (Gn) is one of the envelope proteins on the virus surface and is a major antigenic component. Despite its importance for virus entry and fusion, the molecular features of the phleboviruse Gn were unknown. Here, we present the crystal structures of the Gn head domain from both SFTSV and RVFV, which display a similar compact triangular shape overall, while the three subdomains (domains I, II, and III) making up the Gn head display different arrangements. Ten cysteines in the Gn stem region are conserved among phleboviruses, four of which are responsible for Gn dimerization, as revealed in this study, and they are highly conserved for all members in Bunyaviridae Therefore, we propose an anchoring mode on the viral surface. The complex structure of the SFTSV Gn head and human neutralizing antibody MAb 4-5 reveals that helices α6 in subdomain III is the key component for neutralization. Importantly, the structure indicates that domain III is an ideal region recognized by specific neutralizing antibodies, while domain II is probably recognized by broadly neutralizing antibodies. Collectively, Gn is a desirable vaccine target, and our data provide a molecular basis for the rational design of vaccines against the diseases caused by phleboviruses and a model for bunyavirus Gn embedding on the viral surface.
- Chinese Academy of Sciences Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China.
Organizational Affiliation: