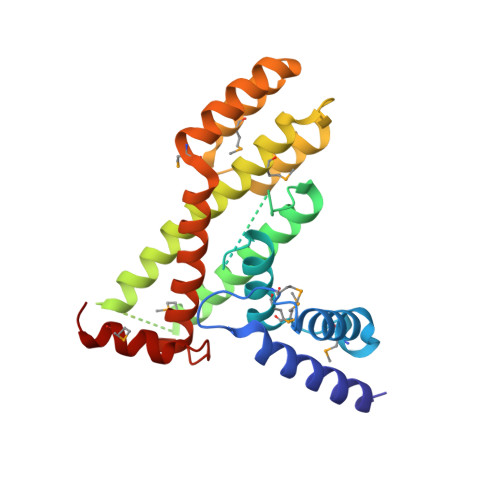
Structure of the fission yeast S. pombe telomeric Tpz1-Poz1-Rap1 complex.
Xue, J., Chen, H., Wu, J., Takeuchi, M., Inoue, H., Liu, Y., Sun, H., Chen, Y., Kanoh, J., Lei, M.(2017) Cell Res 27: 1503-1520
- PubMed: 29160296
- DOI: https://doi.org/10.1038/cr.2017.145
- Primary Citation of Related Structures:
5XXE, 5XXF - PubMed Abstract:
Telomeric shelterin complex caps chromosome ends and plays a crucial role in telomere maintenance and protection. In the fission yeast Schizosaccharomyces pombe, shelterin is composed of telomeric single- and double-stranded DNA-binding protein subcomplexes Pot1-Tpz1 and Taz1-Rap1, which are bridged by their interacting protein Poz1. However, the structure of Poz1 and how Poz1 functions as an interaction hub in the shelterin complex remain unclear. Here we report the crystal structure of Poz1 in complex with Poz1-binding motifs of Tpz1 and Rap1. The crystal structure shows that Poz1 employs two different binding surfaces to interact with Tpz1 and Rap1. Unexpectedly, the structure also reveals that Poz1 adopts a dimeric conformation. Mutational analyses suggest that proper interactions between Tpz1, Poz1, and Rap1 in the shelterin core complex are required for telomere length homeostasis and heterochromatin structure maintenance at telomeres. Structural resemblance between Poz1 and the TRFH domains of other shelterin proteins in fission yeast and humans suggests a model for the evolution of shelterin proteins.
- National Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 333 Haike Road, Shanghai 201210, China.
Organizational Affiliation: