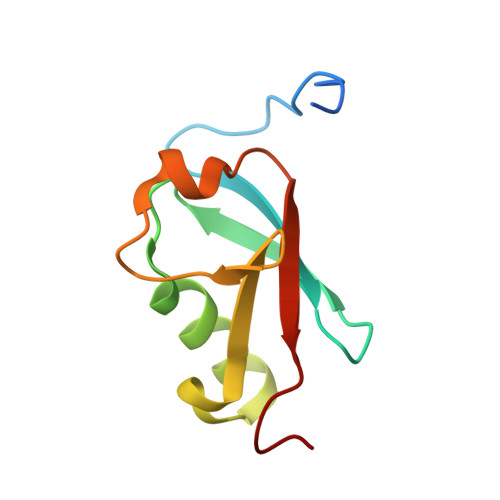
Structural and functional analysis of SMO-1, the SUMO homolog in Caenorhabditis elegans
Surana, P., Gowda, C.M., Tripathi, V., Broday, L., Das, R.(2017) PLoS One 12: e0186622-e0186622
- PubMed: 29045470
- DOI: https://doi.org/10.1371/journal.pone.0186622
- Primary Citation of Related Structures:
5XQM - PubMed Abstract:
SUMO proteins are important post-translational modifiers involved in multiple cellular pathways in eukaryotes, especially during the different developmental stages in multicellular organisms. The nematode C. elegans is a well known model system for studying metazoan development and has a single SUMO homolog, SMO-1. Interestingly, SMO-1 modification is linked to embryogenesis and development in the nematode. However, high-resolution information about SMO-1 and the mechanism of its conjugation is lacking. In this work, we report the high-resolution three dimensional structure of SMO-1 solved by NMR spectroscopy. SMO-1 has flexible N-terminal and C-terminal tails on either side of a rigid beta-grasp folded core. While the sequence of SMO-1 is more similar to SUMO1, the electrostatic surface features of SMO-1 resemble more with SUMO2/3. SMO-1 can bind to typical SUMO Interacting Motifs (SIMs). SMO-1 can also conjugate to a typical SUMOylation consensus site as well as to its natural substrate HMR-1. Poly-SMO-1 chains were observed in-vitro even though SMO-1 lacks any consensus SUMOylation site. Typical deSUMOylation enzymes like Senp2 can cleave the poly-SMO-1 chains. Despite being a single gene, the SMO-1 structure allows it to function in a large repertoire of signaling pathways involving SUMO in C. elegans. Structural and functional features of SMO-1 studies described here will be useful to understand its role in development.
- National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bengaluru, India.
Organizational Affiliation: