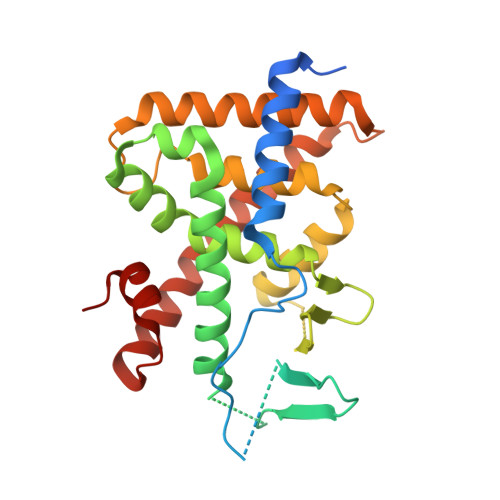
SPA70 is a potent antagonist of human pregnane X receptor.
Lin, W., Wang, Y.M., Chai, S.C., Lv, L., Zheng, J., Wu, J., Zhang, Q., Wang, Y.D., Griffin, P.R., Chen, T.(2017) Nat Commun 8: 741-741
- PubMed: 28963450
- DOI: https://doi.org/10.1038/s41467-017-00780-5
- Primary Citation of Related Structures:
5X0R - PubMed Abstract:
Many drugs bind to and activate human pregnane X receptor (hPXR) to upregulate drug-metabolizing enzymes, resulting in decreased drug efficacy and increased resistance. This suggests that hPXR antagonists have therapeutic value. Here we report that SPA70 is a potent and selective hPXR antagonist. SPA70 inhibits hPXR in human hepatocytes and humanized mouse models and enhances the chemosensitivity of cancer cells, consistent with the role of hPXR in drug resistance. Unexpectedly, SJB7, a close analog of SPA70, is an hPXR agonist. X-ray crystallography reveals that SJB7 resides in the ligand-binding domain (LBD) of hPXR, interacting with the AF-2 helix to stabilize the LBD for coactivator binding. Differential hydrogen/deuterium exchange analysis demonstrates that SPA70 and SJB7 interact with the hPXR LBD. Docking studies suggest that the lack of the para-methoxy group in SPA70 compromises its interaction with the AF-2, thus explaining its antagonism. SPA70 is an hPXR antagonist and promising therapeutic tool.The xenobiotic-activated human pregnane X receptor (hPXR) regulates drug metabolism. Here the authors develop hPXR modulators, which are of potential therapeutic interest and functionally and structurally characterize the antagonist SPA70 and the structurally related agonist SJB7.
- Department of Chemical Biology and Therapeutics, St. Jude Children's Research Hospital, Memphis, TN, 38105-3678, USA.
Organizational Affiliation: