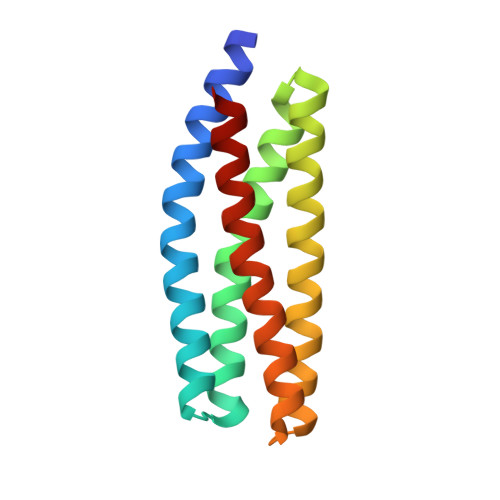
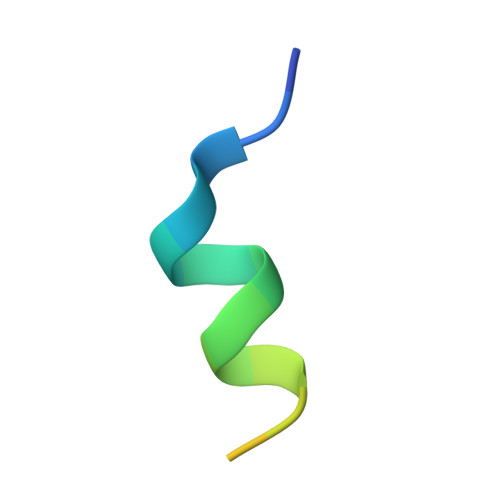
Structural and functional insights into the interaction between the Cas family scaffolding protein p130Cas and the focal adhesion-associated protein paxillin.
Zhang, C., Miller, D.J., Guibao, C.D., Donato, D.M., Hanks, S.K., Zheng, J.J.(2017) J Biological Chem 292: 18281-18289
- PubMed: 28860193
- DOI: https://doi.org/10.1074/jbc.M117.807271
- Primary Citation of Related Structures:
5W93 - PubMed Abstract:
The Cas family scaffolding protein p130Cas is a Src substrate localized in focal adhesions (FAs) and functions in integrin signaling to promote cell motility, invasion, proliferation, and survival. p130Cas targeting to FAs is essential for its tyrosine phosphorylation and downstream signaling. Although the N-terminal SH3 domain is important for p130Cas localization, it has also been reported that the C-terminal region is involved in p130Cas FA targeting. The C-terminal region of p130Cas or Cas family homology domain (CCHD) has been reported to adopt a structure similar to that of the focal adhesion kinase C-terminal focal adhesion-targeting domain. The mechanism by which the CCHD promotes FA targeting of p130Cas, however, remains unclear. In this study, using a calorimetry approach, we identified the first LD motif (LD1) of the FA-associated protein paxillin as the binding partner of the p130Cas CCHD (in a 1:1 stoichiometry with a K d ∼4.2 μm) and elucidated the structure of the p130Cas CCHD in complex with the paxillin LD1 motif by X-ray crystallography. Of note, a comparison of the CCHD/LD1 complex with a previously solved structure of CCHD in complex with the SH2-containing protein NSP3 revealed that LD1 had almost identical positioning of key hydrophobic and acidic residues relative to NSP3. Because paxillin is one of the key scaffold molecules in FAs, we propose that the interaction between the p130Cas CCHD and the LD1 motif of paxillin plays an important role in p130Cas FA targeting.
- From the Stein Eye Institute, Department of Ophthalmology, David Geffen School of Medicine at UCLA, Los Angeles, California 90095.
Organizational Affiliation: