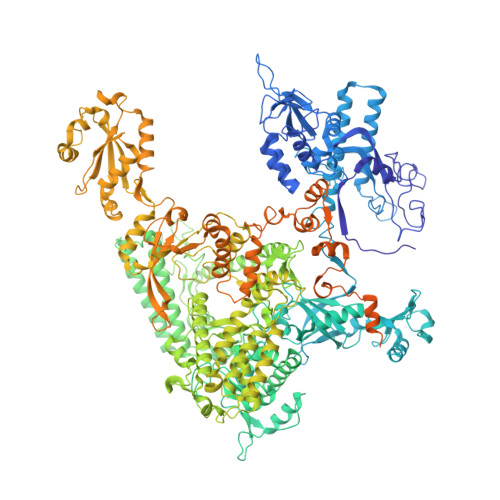
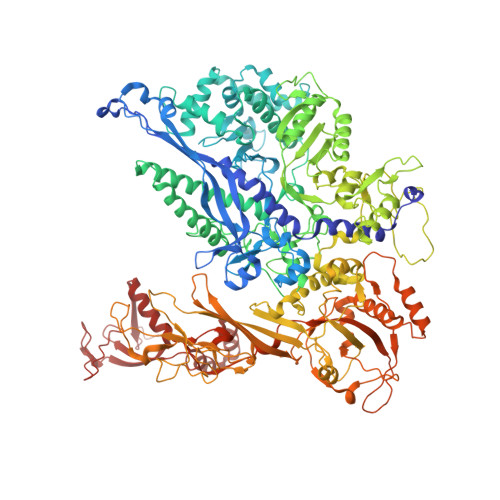
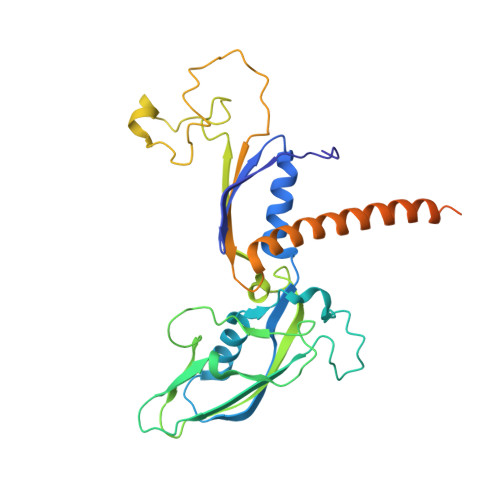
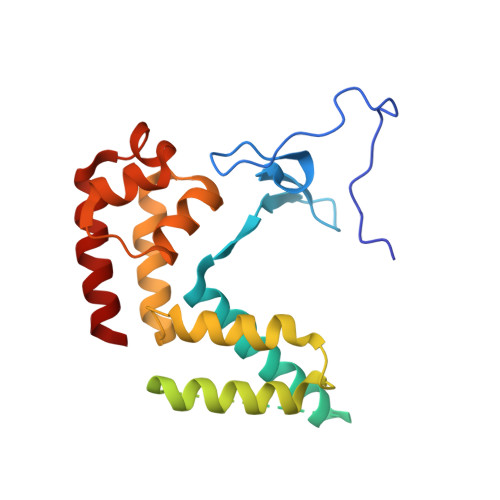
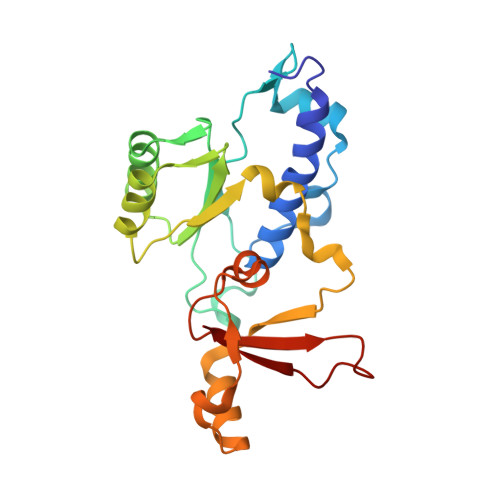
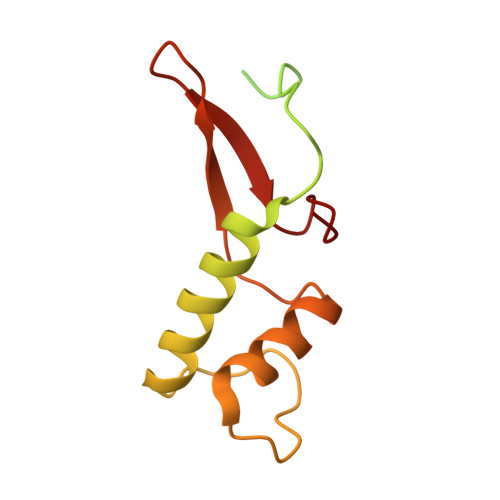
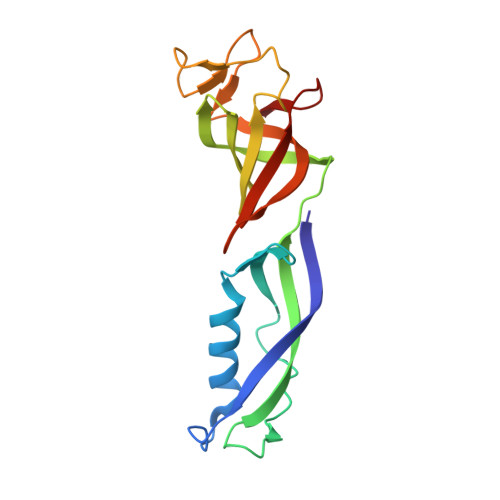
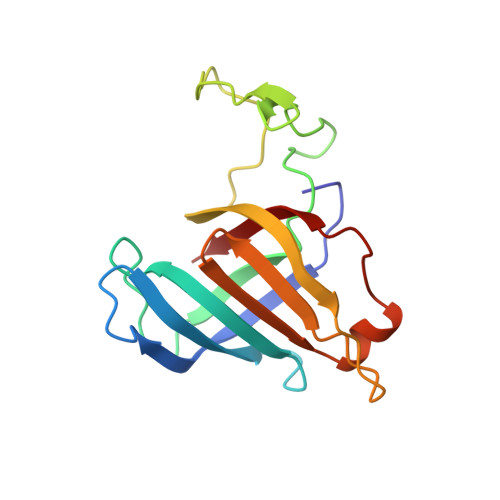
Structural basis for the initiation of eukaryotic transcription-coupled DNA repair.
Xu, J., Lahiri, I., Wang, W., Wier, A., Cianfrocco, M.A., Chong, J., Hare, A.A., Dervan, P.B., DiMaio, F., Leschziner, A.E., Wang, D.(2017) Nature 551: 653-657
- PubMed: 29168508
- DOI: https://doi.org/10.1038/nature24658
- Primary Citation of Related Structures:
5VVR, 5VVS - PubMed Abstract:
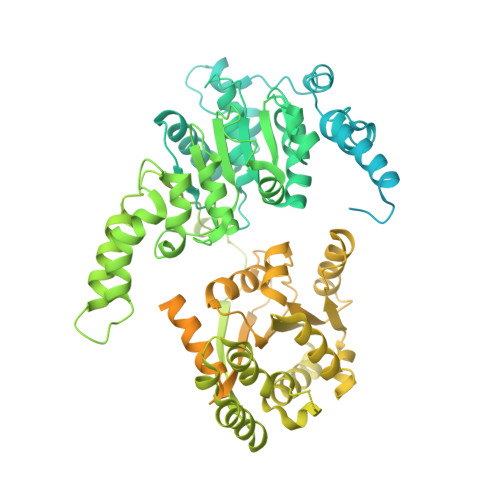
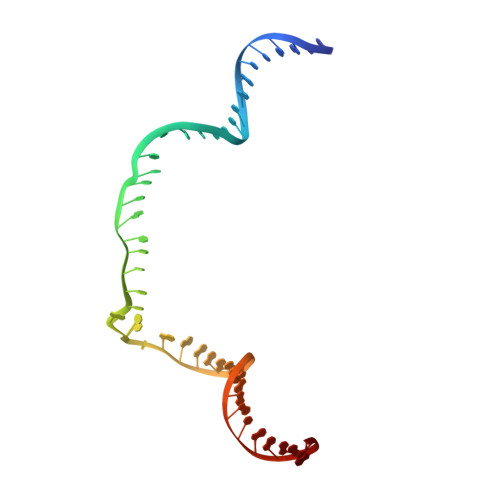
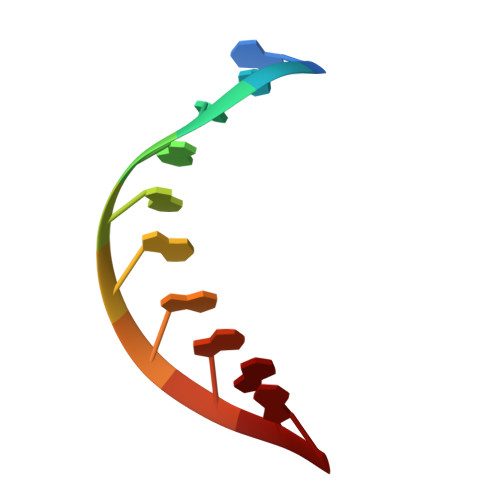
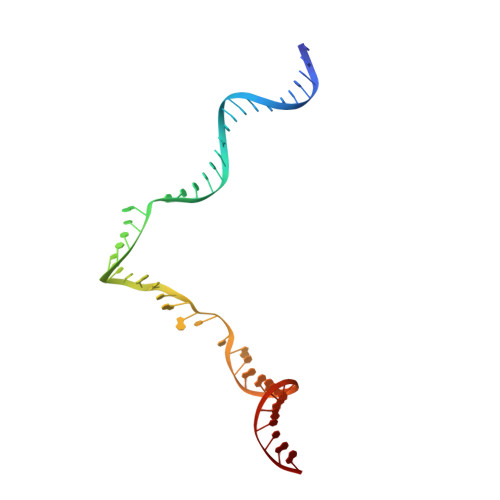
Eukaryotic transcription-coupled repair (TCR) is an important and well-conserved sub-pathway of nucleotide excision repair that preferentially removes DNA lesions from the template strand that block translocation of RNA polymerase II (Pol II). Cockayne syndrome group B (CSB, also known as ERCC6) protein in humans (or its yeast orthologues, Rad26 in Saccharomyces cerevisiae and Rhp26 in Schizosaccharomyces pombe) is among the first proteins to be recruited to the lesion-arrested Pol II during the initiation of eukaryotic TCR. Mutations in CSB are associated with the autosomal-recessive neurological disorder Cockayne syndrome, which is characterized by progeriod features, growth failure and photosensitivity. The molecular mechanism of eukaryotic TCR initiation remains unclear, with several long-standing unanswered questions. How cells distinguish DNA lesion-arrested Pol II from other forms of arrested Pol II, the role of CSB in TCR initiation, and how CSB interacts with the arrested Pol II complex are all unknown. The lack of structures of CSB or the Pol II-CSB complex has hindered our ability to address these questions. Here we report the structure of the S. cerevisiae Pol II-Rad26 complex solved by cryo-electron microscopy. The structure reveals that Rad26 binds to the DNA upstream of Pol II, where it markedly alters its path. Our structural and functional data suggest that the conserved Swi2/Snf2-family core ATPase domain promotes the forward movement of Pol II, and elucidate key roles for Rad26 in both TCR and transcription elongation.
- Division of Pharmaceutical Sciences, Skaggs School of Pharmacy & Pharmaceutical Sciences, University of California San Diego, La Jolla, California 92093, USA.
Organizational Affiliation: