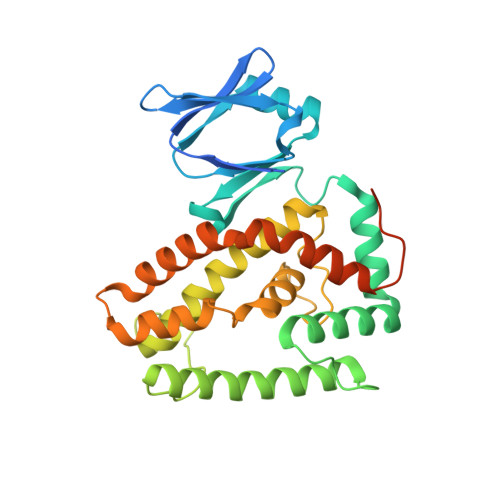
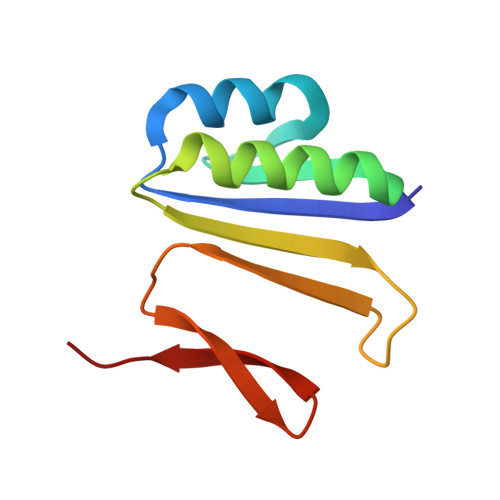
Structures of the CRISPR genome integration complex.
Wright, A.V., Liu, J.J., Knott, G.J., Doxzen, K.W., Nogales, E., Doudna, J.A.(2017) Science 357: 1113-1118
- PubMed: 28729350
- DOI: https://doi.org/10.1126/science.aao0679
- Primary Citation of Related Structures:
5VVJ, 5VVK, 5VVL, 5WFE - PubMed Abstract:
CRISPR-Cas systems depend on the Cas1-Cas2 integrase to capture and integrate short foreign DNA fragments into the CRISPR locus, enabling adaptation to new viruses. We present crystal structures of Cas1-Cas2 bound to both donor and target DNA in intermediate and product integration complexes, as well as a cryo-electron microscopy structure of the full CRISPR locus integration complex, including the accessory protein IHF (integration host factor). The structures show unexpectedly that indirect sequence recognition dictates integration site selection by favoring deformation of the repeat and the flanking sequences. IHF binding bends the DNA sharply, bringing an upstream recognition motif into contact with Cas1 to increase both the specificity and efficiency of integration. These results explain how the Cas1-Cas2 CRISPR integrase recognizes a sequence-dependent DNA structure to ensure site-selective CRISPR array expansion during the initial step of bacterial adaptive immunity.
- Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA.
Organizational Affiliation: