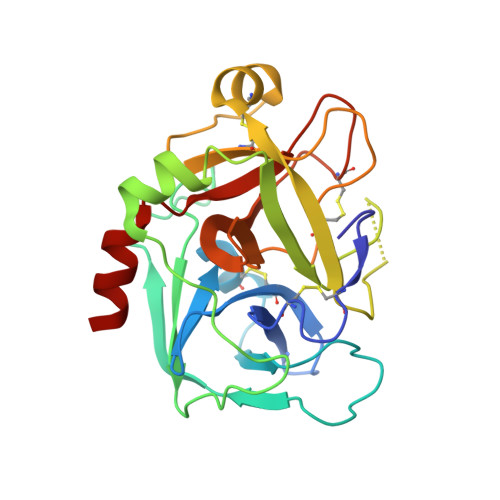
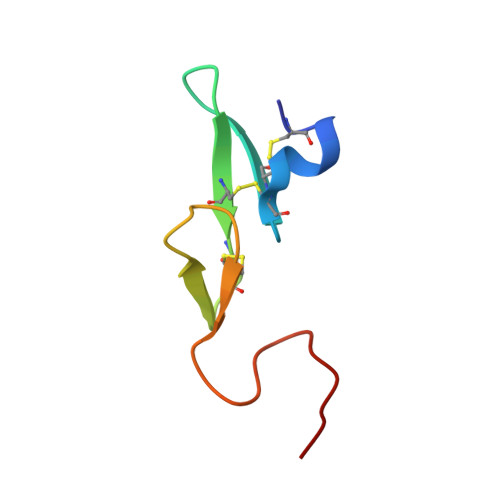
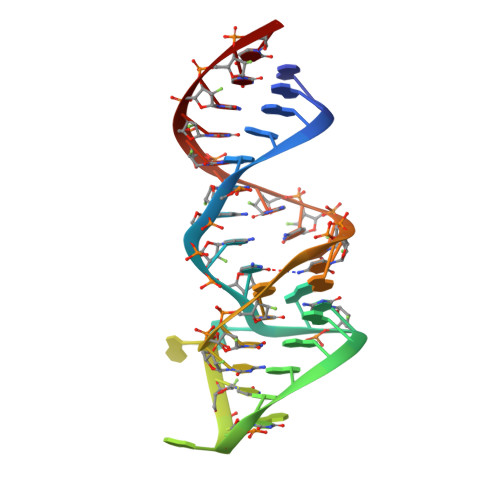
Combination of aptamer and drug for reversible anticoagulation in cardiopulmonary bypass.
Gunaratne, R., Kumar, S., Frederiksen, J.W., Stayrook, S., Lohrmann, J.L., Perry, K., Bompiani, K.M., Chabata, C.V., Thalji, N.K., Ho, M.D., Arepally, G., Camire, R.M., Krishnaswamy, S., Sullenger, B.A.(2018) Nat Biotechnol 36: 606-613
- PubMed: 29863725
- DOI: https://doi.org/10.1038/nbt.4153
- Primary Citation of Related Structures:
5VOE, 5VOF - PubMed Abstract:
Unfractionated heparin (UFH), the standard anticoagulant for cardiopulmonary bypass (CPB) surgery, carries a risk of post-operative bleeding and is potentially harmful in patients with heparin-induced thrombocytopenia-associated antibodies. To improve the activity of an alternative anticoagulant, the RNA aptamer 11F7t, we solved X-ray crystal structures of the aptamer bound to factor Xa (FXa). The finding that 11F7t did not bind the catalytic site suggested that it could complement small-molecule FXa inhibitors. We demonstrate that combinations of 11F7t and catalytic-site FXa inhibitors enhance anticoagulation in purified reaction mixtures and plasma. Aptamer-drug combinations prevented clot formation as effectively as UFH in human blood circulated in an extracorporeal oxygenator circuit that mimicked CPB, while avoiding side effects of UFH. An antidote could promptly neutralize the anticoagulant effects of both FXa inhibitors. Our results suggest that drugs and aptamers with shared targets can be combined to exert more specific and potent effects than either agent alone.
- Department of Pharmacology and Cancer Biology, Duke University, Durham, North Carolina, USA.
Organizational Affiliation: