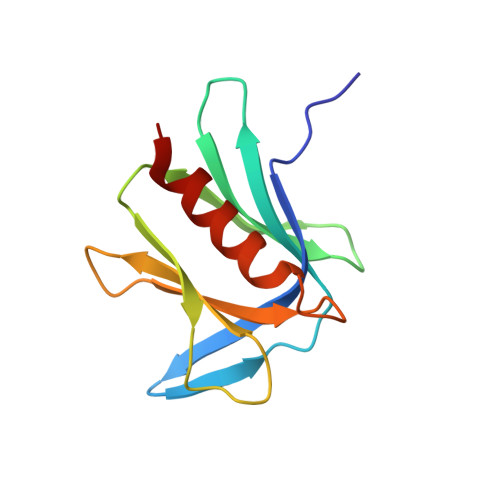
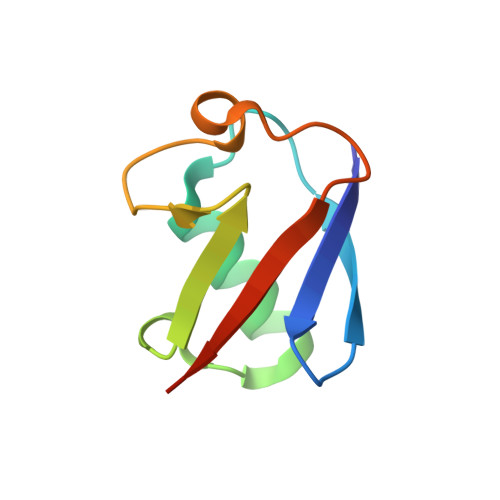
Structure and energetics of pairwise interactions between proteasome subunits RPN2, RPN13, and ubiquitin clarify a substrate recruitment mechanism.
VanderLinden, R.T., Hemmis, C.W., Yao, T., Robinson, H., Hill, C.P.(2017) J Biological Chem 292: 9493-9504
- PubMed: 28442575
- DOI: https://doi.org/10.1074/jbc.M117.785287
- Primary Citation of Related Structures:
5V1Y, 5V1Z - PubMed Abstract:
The 26S proteasome is a large cellular assembly that mediates the selective degradation of proteins in the nucleus and cytosol and is an established target for anticancer therapeutics. Protein substrates are typically targeted to the proteasome through modification with a polyubiquitin chain, which can be recognized by several proteasome-associated ubiquitin receptors. One of these receptors, RPN13/ADRM1, is recruited to the proteasome through direct interaction with the large scaffolding protein RPN2 within the 19S regulatory particle. To better understand the interactions between RPN13, RPN2, and ubiquitin, we used human proteins to map the RPN13-binding epitope to the C-terminal 14 residues of RPN2, which, like ubiquitin, binds the N-terminal pleckstrin-like receptor of ubiquitin (PRU) domain of RPN13. We also report the crystal structures of the RPN13 PRU domain in complex with peptides corresponding to the RPN2 C terminus and ubiquitin. Through mutational analysis, we validated the RPN2-binding interface revealed by our structures and quantified binding interactions with surface plasmon resonance and fluorescence polarization. In contrast to a previous report, we find that RPN13 binds ubiquitin with an affinity similar to that of other proteasome-associated ubiquitin receptors and that RPN2, ubiquitin, and the deubiquitylase UCH37 bind to RPN13 with independent energetics. These findings provide a detailed characterization of interactions that are important for proteasome function, indicate ubiquitin affinities that are consistent with the role of RPN13 as a proteasomal ubiquitin receptor, and have major implications for the development of novel anticancer therapeutics.
- From the Department of Biochemistry, University of Utah, Salt Lake City, Utah 84112.
Organizational Affiliation: