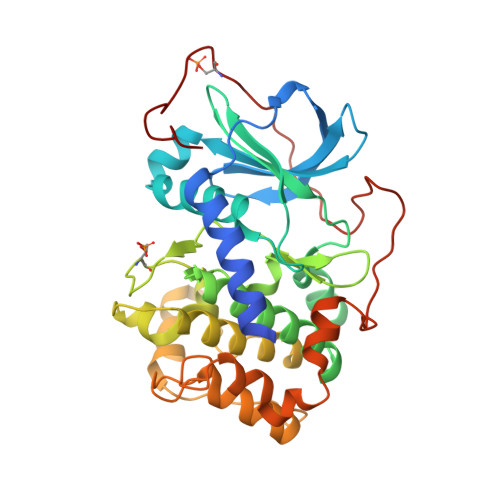
ROCK inhibitors 3: Design, synthesis and structure-activity relationships of 7-azaindole-based Rho kinase (ROCK) inhibitors.
Bandarage, U.K., Cao, J., Come, J.H., Court, J.J., Gao, H., Jacobs, M.D., Marhefka, C., Nanthakumar, S., Green, J.(2018) Bioorg Med Chem Lett 28: 2622-2626
- PubMed: 30082069
- DOI: https://doi.org/10.1016/j.bmcl.2018.06.040
- Primary Citation of Related Structures:
5UZJ, 5UZK - PubMed Abstract:
Rho kinase (ROCK) inhibitors are potential therapeutic agents for the treatment of a variety of disorders including hypertension, glaucoma and erectile dysfunction. Here we disclose a series of potent and selective ROCK inhibitors based on a substituted 7-azaindole scaffold. Substitution of the 3-position of 7-azaindole led to compounds such as 37, which possess excellent ROCK inhibitory potency and high selectivity against the closely related kinase PKA.
- Vertex Pharmaceuticals Incorporated, 50 Northern Avenue, Boston, MA 02210, USA. Electronic address: upul_bandarage@vrtx.com.
Organizational Affiliation: