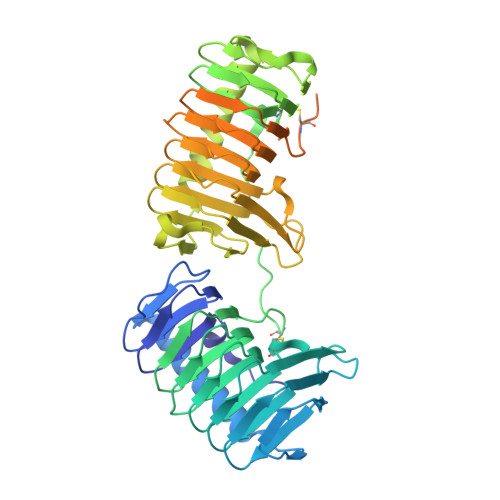
Structural basis of antifreeze activity of a bacterial multi-domain antifreeze protein.
Wang, C., Pakhomova, S., Newcomer, M.E., Christner, B.C., Luo, B.H.(2017) PLoS One 12: e0187169-e0187169
- PubMed: 29108002
- DOI: https://doi.org/10.1371/journal.pone.0187169
- Primary Citation of Related Structures:
5UYT - PubMed Abstract:
Antifreeze proteins (AFPs) enhance the survival of organisms inhabiting cold environments by affecting the formation and/or structure of ice. We report the crystal structure of the first multi-domain AFP that has been characterized. The two ice binding domains are structurally similar. Each consists of an irregular β-helix with a triangular cross-section and a long α-helix that runs parallel on one side of the β-helix. Both domains are stabilized by hydrophobic interactions. A flat plane on the same face of each domain's β-helix was identified as the ice binding site. Mutating any of the smaller residues on the ice binding site to bulkier ones decreased the antifreeze activity. The bulky side chain of Leu174 in domain A sterically hinders the binding of water molecules to the protein backbone, partially explaining why antifreeze activity by domain A is inferior to that of domain B. Our data provide a molecular basis for understanding differences in antifreeze activity between the two domains of this protein and general insight on how structural differences in the ice-binding sites affect the activity of AFPs.
- Department of Biological Sciences, Louisiana State University, Baton Rouge, Louisiana, United States of America.
Organizational Affiliation: