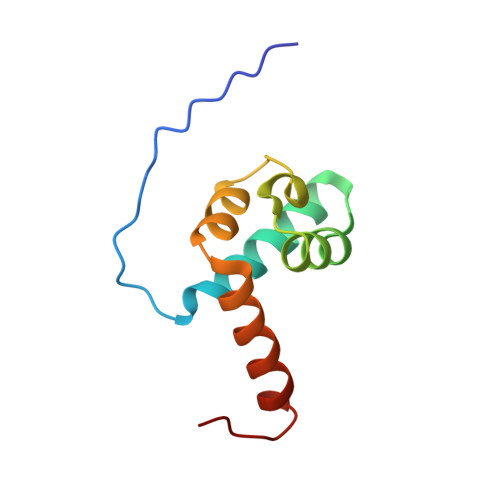
Structure and Dimerization of IreB, a Negative Regulator of Cephalosporin Resistance in Enterococcus faecalis.
Hall, C.L., Lytle, B.L., Jensen, D., Hoff, J.S., Peterson, F.C., Volkman, B.F., Kristich, C.J.(2017) J Mol Biology 429: 2324-2336
- PubMed: 28551334
- DOI: https://doi.org/10.1016/j.jmb.2017.05.019
- Primary Citation of Related Structures:
5US5 - PubMed Abstract:
Enterococcus faecalis, a leading cause of hospital-acquired infections, exhibits intrinsic resistance to most cephalosporins, which are antibiotics in the beta-lactam family that target cell-wall biosynthesis. A comprehensive understanding of the underlying genetic and biochemical mechanisms of cephalosporin resistance in E. faecalis is lacking. We previously determined that a transmembrane serine/threonine kinase (IreK) and its cognate phosphatase (IreP) reciprocally regulate cephalosporin resistance in E. faecalis, dependent on the kinase activity of IreK. Other than IreK itself, thus far the only known substrate for reversible phosphorylation by IreK and IreP is IreB, a small protein of unknown function that is well conserved in low-GC Gram-positive bacteria. We previously showed that IreB acts as a negative regulator of cephalosporin resistance in E. faecalis. However, the biochemical mechanism by which IreB modulates cephalosporin resistance remains unknown. As a next step toward an understanding of the mechanism by which IreB regulates resistance, we initiated a structure-function study on IreB. The NMR solution structure of IreB was determined, revealing that IreB adopts a unique fold and forms a dimer in vitro. Dimerization of IreB was confirmed in vivo. Substitutions at the dimer interface impaired IreB function and stability in vivo, indicating that dimerization is functionally important for the biological activity of IreB. Hence, these studies provide new insights into the structure and function of a widely conserved protein of unknown function that is an important regulator of antimicrobial resistance in E. faecalis.
- Department of Microbiology and Immunology, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Center for Infectious Disease Research, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Organizational Affiliation: