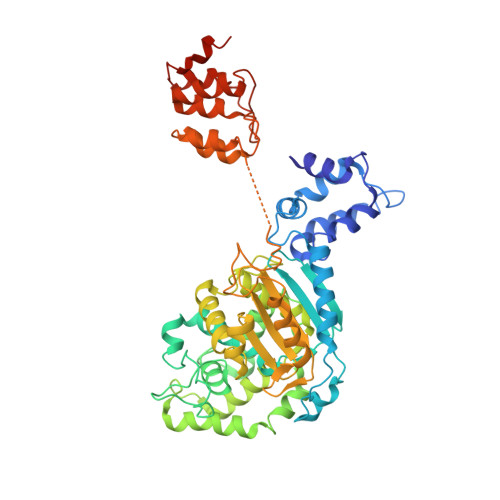
The origin and evolution of human glutaminases and their atypical C-terminal ankyrin repeats.
Pasquali, C.C., Islam, Z., Adamoski, D., Ferreira, I.M., Righeto, R.D., Bettini, J., Portugal, R.V., Yue, W.W., Gonzalez, A., Dias, S.M.G., Ambrosio, A.L.B.(2017) J Biological Chem 292: 11572-11585
- PubMed: 28526749
- DOI: https://doi.org/10.1074/jbc.M117.787291
- Primary Citation of Related Structures:
5U0I, 5U0J, 5U0K, 5UQE - PubMed Abstract:
On the basis of tissue-specific enzyme activity and inhibition by catalytic products, Hans Krebs first demonstrated the existence of multiple glutaminases in mammals. Currently, two human genes are known to encode at least four glutaminase isoforms. However, the phylogeny of these medically relevant enzymes remains unclear, prompting us to investigate their origin and evolution. Using prokaryotic and eukaryotic glutaminase sequences, we built a phylogenetic tree whose topology suggested that the multidomain architecture was inherited from bacterial ancestors, probably simultaneously with the hosting of the proto-mitochondrion endosymbiont. We propose an evolutionary model wherein the appearance of the most active enzyme isoform, glutaminase C (GAC), which is expressed in many cancers, was a late retrotransposition event that occurred in fishes from the Chondrichthyes class. The ankyrin (ANK) repeats in the glutaminases were acquired early in their evolution. To obtain information on ANK folding, we solved two high-resolution structures of the ANK repeat-containing C termini of both kidney-type glutaminase (KGA) and GLS2 isoforms (glutaminase B and liver-type glutaminase). We found that the glutaminase ANK repeats form unique intramolecular contacts through two highly conserved motifs; curiously, this arrangement occludes a region usually involved in ANK-mediated protein-protein interactions. We also solved the crystal structure of full-length KGA and present a small-angle X-ray scattering model for full-length GLS2. These structures explain these proteins' compromised ability to assemble into catalytically active supra-tetrameric filaments, as previously shown for GAC. Collectively, these results provide information about glutaminases that may aid in the design of isoform-specific glutaminase inhibitors.
- From the Laboratório Nacional de Biociências, Centro Nacional de Pesquisa em Energia e Materiais, Campinas, São Paulo 13083-970, Brazil.
Organizational Affiliation: