Design of ultrahigh-affinity and dual-specificity peptide antagonists of MDM2 and MDMX for p53 activation
Pazgier, M., Gohain, N., Tolbert, W.D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein Mdm4 | A, C [auth B], E, G | 85 | Homo sapiens | Mutation(s): 3 | 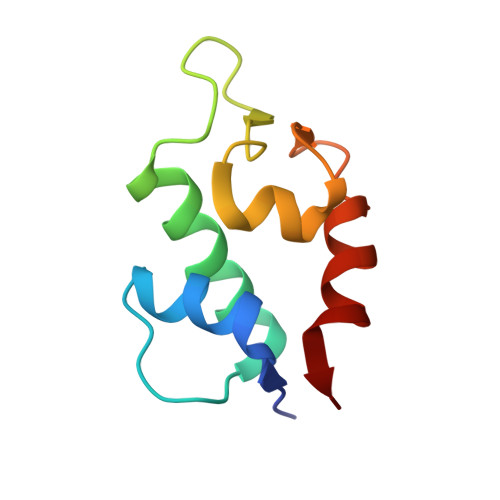 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15151 (Homo sapiens) Explore O15151 Go to UniProtKB: O15151 | |||||
PHAROS: O15151 GTEx: ENSG00000198625 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15151 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PEPTIDE INHIBITOR M3 | B [auth C], D, F, H | 12 | synthetic construct | Mutation(s): 0 | 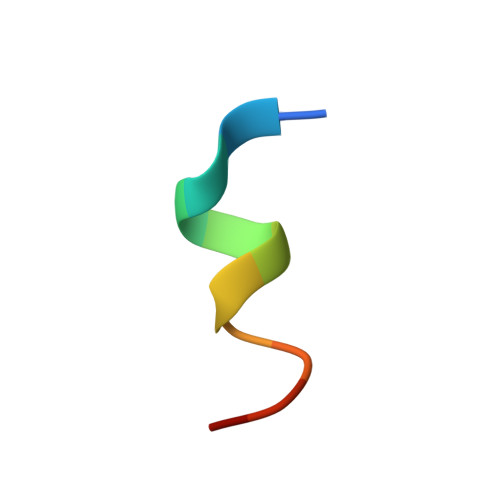 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 45.413 | α = 90 |
| b = 88.027 | β = 90.66 |
| c = 46.3 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PHASER | phasing |
| HKL-2000 | data scaling |
| HKL-2000 | data reduction |