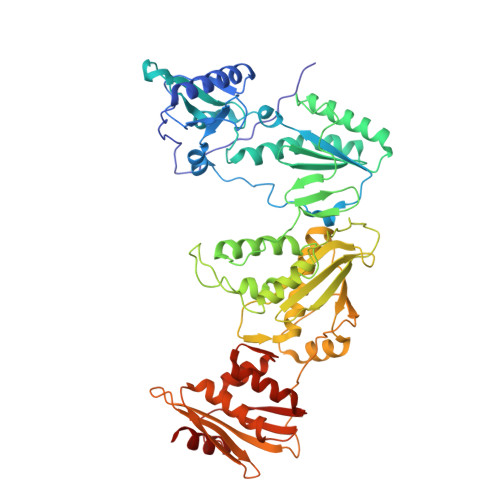
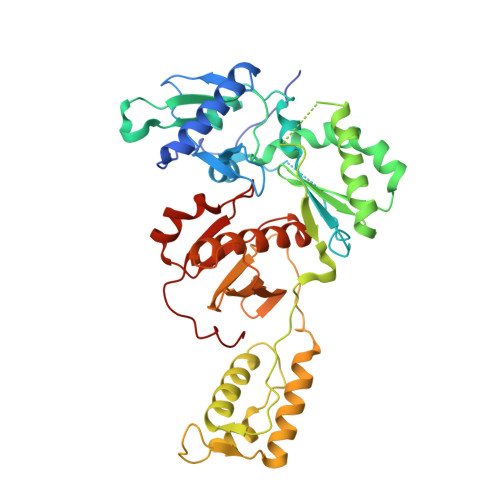
6-Cyclohexylmethyl-3-hydroxypyrimidine-2,4-dione as an inhibitor scaffold of HIV reverase transcriptase: Impacts of the 3-OH on inhibiting RNase H and polymerase.
Tang, J., Kirby, K.A., Huber, A.D., Casey, M.C., Ji, J., Wilson, D.J., Sarafianos, S.G., Wang, Z.(2017) Eur J Med Chem 128: 168-179
- PubMed: 28182989
- DOI: https://doi.org/10.1016/j.ejmech.2017.01.041
- Primary Citation of Related Structures:
5TUQ - PubMed Abstract:
3-Hydroxypyrimidine-2,4-dione (HPD) represents a versatile chemical core in the design of inhibitors of human immunodeficiency virus (HIV) reverse transcriptase (RT)-associated RNase H and integrase strand transfer (INST). We report herein the design, synthesis and biological evaluation of an HPD subtype (4) featuring a cyclohexylmethyl group at the C-6 position. Antiviral testing showed that most analogues of 4 inhibited HIV-1 in the low nanomolar to submicromolar range, without cytotoxicity at concentrations up to 100 μM. Biochemically, these analogues dually inhibited both the polymerase (pol) and the RNase H functions of RT, but not INST. Co-crystal structure of 4a with RT revealed a nonnucleoside RT inhibitor (NNRTI) binding mode. Interestingly, chemotype 11, the synthetic precursor of 4 lacking the 3-OH group, did not inhibit RNase H while potently inhibiting pol. By virtue of the potent antiviral activity and biochemical RNase H inhibition, HPD subtype 4 could provide a viable platform for eventually achieving potent and selective RNase H inhibition through further medicinal chemistry.
- Center for Drug Design, Academic Health Center, University of Minnesota, Minneapolis, MN 55455, USA.
Organizational Affiliation: