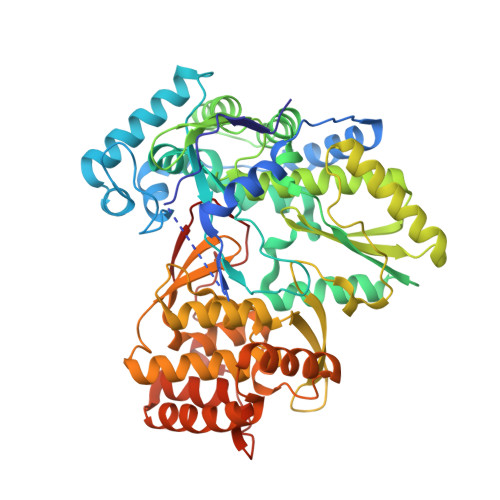
Discovery and initial optimization of alkoxyanthranilic acid derivatives as inhibitors of HCV NS5B polymerase.
Parcella, K., Nickel, A., Beno, B.R., Sheriff, S., Wan, C., Wang, Y.K., Roberts, S.B., Meanwell, N.A., Kadow, J.F.(2017) Bioorg Med Chem Lett 27: 295-298
- PubMed: 27908764
- DOI: https://doi.org/10.1016/j.bmcl.2016.11.054
- Primary Citation of Related Structures:
5TRH, 5TRI, 5TRJ, 5TRK - PubMed Abstract:
Alkoxyanthranilic acid derivatives have been identified to inhibit HCV NS5B polymerase, binding in an allosteric site located at the convergence of the palm and thumb regions. Information from co-crystal structures guided the structural design strategy. Ultimately, two independent structural modifications led to a similar shift in binding mode that when combined led to a synergistic improvement in potency and the identification of inhibitors with sub-micromolar HCV NS5B binding potency.
- Bristol-Myers Squibb, Research and Development, 5 Research Parkway, Wallingford, CT 06492, USA. Electronic address: kyle.parcella@bms.com.
Organizational Affiliation: