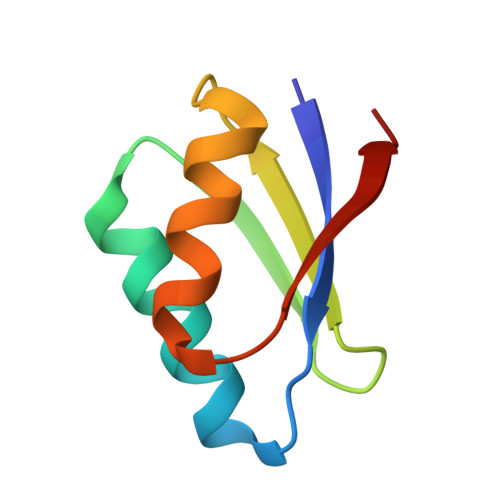
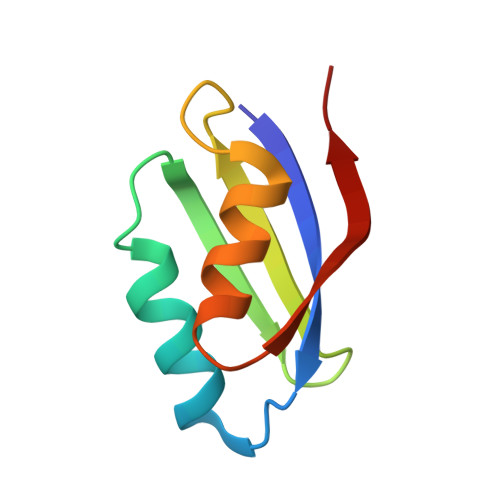
Mechanistic and Structural Basis for Inhibition of Copper Trafficking by Platinum Anticancer Drugs.
Lasorsa, A., Nardella, M.I., Rosato, A., Mirabelli, V., Caliandro, R., Caliandro, R., Natile, G., Arnesano, F.(2019) J Am Chem Soc 141: 12109-12120
- PubMed: 31283225
- DOI: https://doi.org/10.1021/jacs.9b05550
- Primary Citation of Related Structures:
5T7L - PubMed Abstract:
Copper (Cu) is required for maturation of cuproenzymes, cell proliferation, and angiogenesis, and its transport entails highly specific protein-protein interactions. In humans, the Cu chaperone Atox1 mediates Cu(I) delivery to P-type ATPases Atp7a and Atp7b (the Menkes and Wilson disease proteins, respectively), which are responsible for Cu release to the secretory pathway and excess Cu efflux. Cu(I) handover is believed to occur through the formation of three-coordinate intermediates where the metal ion is simultaneously linked to Atox1 and to a soluble domain of Cu-ATPases, both sharing a CxxC dithiol motif. The ultrahigh thermodynamic stability of chelating S-donor ligands secures the redox-active and potentially toxic Cu(I) ion, while their kinetic lability allows facile metal transfer. The same CxxC motifs can interact with and mediate the biological response to antitumor platinum drugs, which are among the most used chemotherapeutics. We show that cisplatin and an oxaliplatin analogue can specifically bind to the heterodimeric complex Atox1-Cu(I)-Mnk1 (Mnk1 is the first soluble domain of Atp7a), thus leading to a kinetically stable adduct that has been structurally characterized by solution NMR and X-ray crystallography. Of the two possible binding configurations of the Cu(I) ion in the cage made by the CxxC motifs of the two proteins, one (bidentate Atox1 and monodentate Mnk1) is less stable and more reactive toward cis -Pt(II) compounds, as shown by using mutated proteins. A Cu(I) ion can be retained at the Pt(II) coordination site but can be released to glutathione (a physiological thiol) or to other complexing agents. The Pt(II)-supported heterodimeric complex does not form if Zn(II) is used in place of Cu(I) and transplatin instead of cisplatin. The results indicate that Pt(II) drugs can specifically affect Cu(I) homeostasis by interfering with the rapid exchange of Cu(I) between Atox1 and Cu-ATPases with consequences on cancer cell viability and migration.
- Department of Chemistry , University of Bari "Aldo Moro" , via Orabona, 4 , 70125 Bari , Italy.
Organizational Affiliation: