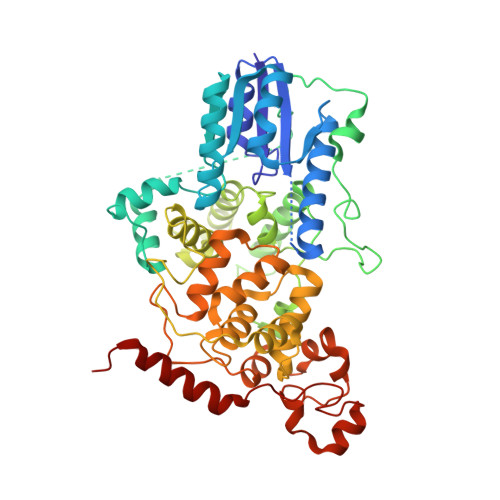
Formation of a repressive complex in the mammalian circadian clock is mediated by the secondary pocket of CRY1.
Michael, A.K., Fribourgh, J.L., Chelliah, Y., Sandate, C.R., Hura, G.L., Schneidman-Duhovny, D., Tripathi, S.M., Takahashi, J.S., Partch, C.L.(2017) Proc Natl Acad Sci U S A 114: 1560-1565
- PubMed: 28143926
- DOI: https://doi.org/10.1073/pnas.1615310114
- Primary Citation of Related Structures:
5T5X - PubMed Abstract:
The basic helix-loop-helix PAS domain (bHLH-PAS) transcription factor CLOCK:BMAL1 (brain and muscle Arnt-like protein 1) sits at the core of the mammalian circadian transcription/translation feedback loop. Precise control of CLOCK:BMAL1 activity by coactivators and repressors establishes the ∼24-h periodicity of gene expression. Formation of a repressive complex, defined by the core clock proteins cryptochrome 1 (CRY1):CLOCK:BMAL1, plays an important role controlling the switch from repression to activation each day. Here we show that CRY1 binds directly to the PAS domain core of CLOCK:BMAL1, driven primarily by interaction with the CLOCK PAS-B domain. Integrative modeling and solution X-ray scattering studies unambiguously position a key loop of the CLOCK PAS-B domain in the secondary pocket of CRY1, analogous to the antenna chromophore-binding pocket of photolyase. CRY1 docks onto the transcription factor alongside the PAS domains, extending above the DNA-binding bHLH domain. Single point mutations at the interface on either CRY1 or CLOCK disrupt formation of the ternary complex, highlighting the importance of this interface for direct regulation of CLOCK:BMAL1 activity by CRY1.
- Department of Chemistry and Biochemistry, University of California, Santa Cruz, CA 95064.
Organizational Affiliation: