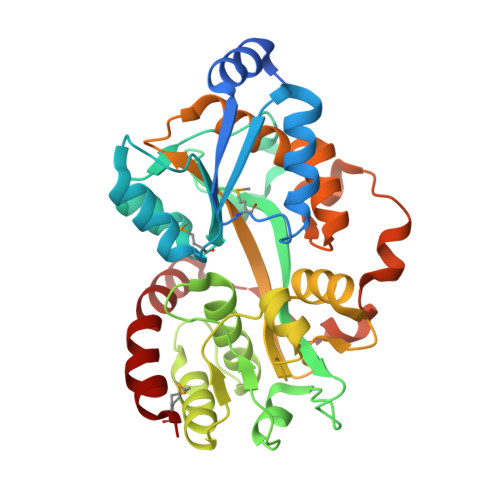
Crystal structure of the putative periplasmic solute-binding protein from Campylobacter jejuni
Filippova, E.V., Wawrzsak, Z., Sandoval, J., Skarina, T., Grimshaw, S., Savchenko, A., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.