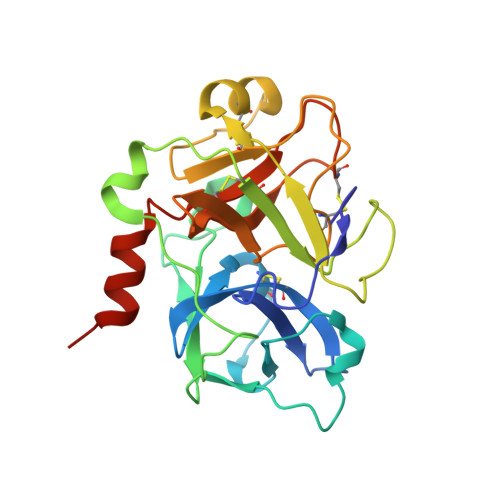
Potent, Orally Bioavailable and Efficacious Macrocyclic Inhibitors of Factor XIa. Discovery of Pyridine-Based Macrocycles Possessing Phenylazole Carboxamide P1 Groups.
Corte, J.R., Pinto, D.J.P., Fang, T., Osuna, H., Yang, W., Wang, Y., Lai, A., Clark, C., Sun, J.H., Rampulla, R.A., Mathur, A., Kaspady, M., Neithnadka, P.R., Li, Y.X., Rossi, K.A., Myers, J.E., Sheriff, S., Lou, Z., Harper, T.W., Huang, C.S., Zheng, J.J., Bozarth, J.M., Wu, Y., Wong, P.C., Crain, E., Seiffert, D.A., Luettgen, J.M., Lam, P., Wexler, R.R., Ewing, W.R.(2019) J Med Chem 63: 784-803
- PubMed: 31833761
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01768
- Primary Citation of Related Structures:
5QTT, 5QTU - PubMed Abstract:
Factor XIa (FXIa) inhibitors are promising novel anticoagulants, which show excellent efficacy in preclinical thrombosis models with minimal effects on hemostasis. The discovery of potent and selective FXIa inhibitors which are also orally bioavailable has been a challenge. Here, we describe optimization of the imidazole-based macrocyclic series and our initial progress toward meeting this challenge. A two-pronged strategy, which focused on replacement of the imidazole scaffold and the design of new P1 groups, led to the discovery of potent, orally bioavailable pyridine-based macrocyclic FXIa inhibitors. Moreover, pyridine-based macrocycle 19 , possessing the phenylimidazole carboxamide P1, exhibited excellent selectivity against relevant blood coagulation enzymes and displayed antithrombotic efficacy in a rabbit thrombosis model.
- Research and Development , Bristol-Myers Squibb Company , 350 Carter Road , Hopewell , New Jersey 08540 , United States.
Organizational Affiliation: