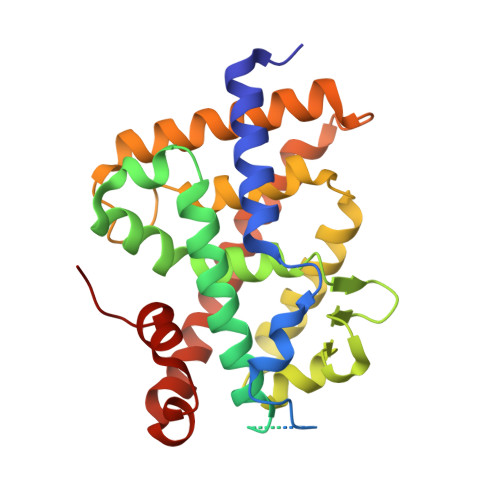
Investigation of 20S-hydroxyvitamin D3 analogs and their 1 alpha-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities.
Lin, Z., Marepally, S.R., Goh, E.S.Y., Cheng, C.Y.S., Janjetovic, Z., Kim, T.K., Miller, D.D., Postlethwaite, A.E., Slominski, A.T., Tuckey, R.C., Peluso-Iltis, C., Rochel, N., Li, W.(2018) Sci Rep 8: 1478-1478
- PubMed: 29367669
- DOI: https://doi.org/10.1038/s41598-018-19183-7
- Primary Citation of Related Structures:
5OW7, 5OW9, 5OWD - PubMed Abstract:
20S-hydroxyvitamin D 3 [20S(OH)D 3 ] is anti-inflammatory and not hypercalcemic, suggesting its potential as a lead compound. In this study, side chain modified 20S(OH)D 3 analogs (4, 13, 23 and 33) together with their 1α-OH derivatives were synthesized and their metabolism and biological activities tested. 4, 13 and 23 are good substrates for CYP27B1, enabling enzymatic synthesis of their 1α-OH derivatives 5, 14 and 24. However, 33 could not be hydroxylated by CYP27B1 and acts as an inhibitor. All analogs were poorer substrates for CYP24A1 than calcitriol, indicating improved catabolic stability. While the parent analogs showed minimal VDR stimulating activity, their 1α-OH derivatives were potent VDR agonists. 4, 5, 14 and 24 significantly upregulated the expression of CYP24A1 at the mRNA level, consistent with their VDR activation abilities and indicating that 1α-hydroxylation is required to produce analogs with strong activity. These analogs have anti-inflammatory activities that are influenced by side chain composition and by 1α-hydroxylation. To understand their molecular interactions with the VDR, 20S(OH)D 3 , 4 and 33 were co-crystalized with the VDR ligand binding domain, which revealed subtle differences to the calcitriol-bound receptor. This study demonstrates the potential of the 20S(OH)D 3 scaffold for the development of novel anti-inflammatory agents.
- Department of Pharmaceutical Sciences, University of Tennessee Health Science Center, Memphis, TN, 38163, United States.
Organizational Affiliation: