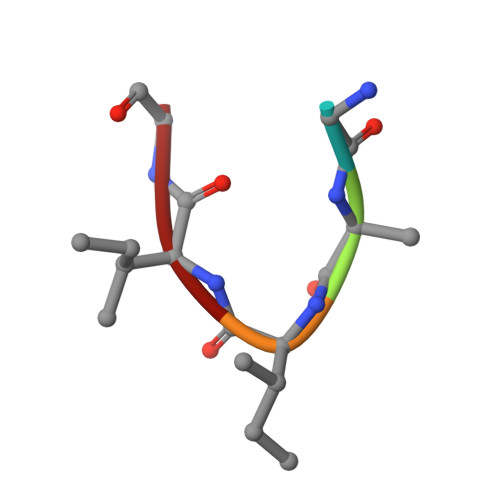
Alzheimer's A beta1-40peptide degradation by thermolysin: evidence of inhibition by a C-terminal A beta product.
Leite, J.P., Gales, L.(2019) FEBS Lett 593: 128-137
- PubMed: 30403288
- DOI: https://doi.org/10.1002/1873-3468.13285
- Primary Citation of Related Structures:
5ONP, 5ONQ, 5ONR, 6GHX - PubMed Abstract:
The interaction of the amyloid-β peptide (Aβ) with thermolysin (TLN) was investigated by X-ray crystallography. Structural models of the complexes of TLN with several Aβ fragments show that, despite the numerous possible cleavage sites of the Aβ sequence, the C-terminal product of Ala30-Ile31 cleavage does not dissociate, thus inhibiting the enzyme. The high similarity between the TLN structural motif and neprilysin (NEP), the most extensively studied peptidase associated with Aβ clearance, suggests that NEP should be more efficient against Aβ polymorphs where Ala30-Ile31 is inaccessible, which is in agreement with studies in living mice that point to the limited role of NEP in degrading soluble Aβ and its higher ability to degrade insoluble and/or oligomeric Aβ forms, producing only the Aβ 10-37 intermediate.
- i3S - Instituto de Investigação e Inovação em Saúde, Porto, Portugal.
Organizational Affiliation: