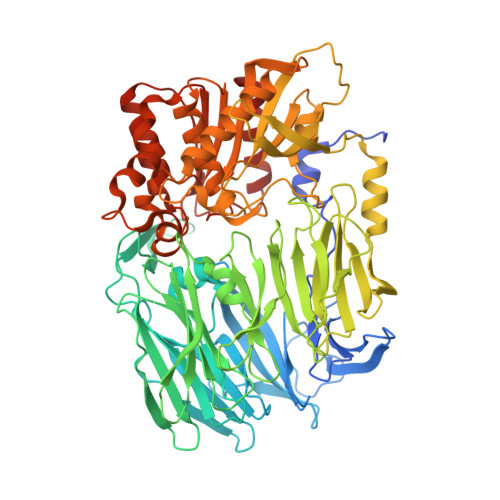
Crystal structure of Porphyromonas gingivalis dipeptidyl peptidase 4 and structure-activity relationships based on inhibitor profiling.
Rea, D., Van Elzen, R., De Winter, H., Van Goethem, S., Landuyt, B., Luyten, W., Schoofs, L., Van Der Veken, P., Augustyns, K., De Meester, I., Fulop, V., Lambeir, A.M.(2017) Eur J Med Chem 139: 482-491
- PubMed: 28826083
- DOI: https://doi.org/10.1016/j.ejmech.2017.08.024
- Primary Citation of Related Structures:
5OLJ - PubMed Abstract:
The Gram-negative anaerobe Porphyromonas gingivalis is associated with chronic periodontitis. Clinical isolates of P. gingivalis strains with high dipeptidyl peptidase 4 (DPP4) expression also had a high capacity for biofilm formation and were more infective. The X-ray crystal structure of P. gingivalis DPP4 was solved at 2.2 Å resolution. Despite a sequence identity of 32%, the overall structure of the dimer was conserved between P. gingivalis DPP4 and mammalian orthologues. The structures of the substrate binding sites were also conserved, except for the region called S2-extensive, which is exploited by specific human DPP4 inhibitors currently used as antidiabetic drugs. Screening of a collection of 450 compounds as inhibitors revealed a structure-activity relationship that mimics in part that of mammalian DPP9. The functional similarity between human and bacterial DPP4 was confirmed using 124 potential peptide substrates.
- School of Life Sciences, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK.
Organizational Affiliation: