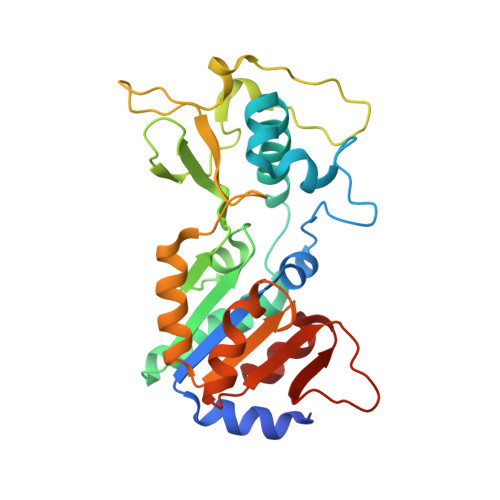
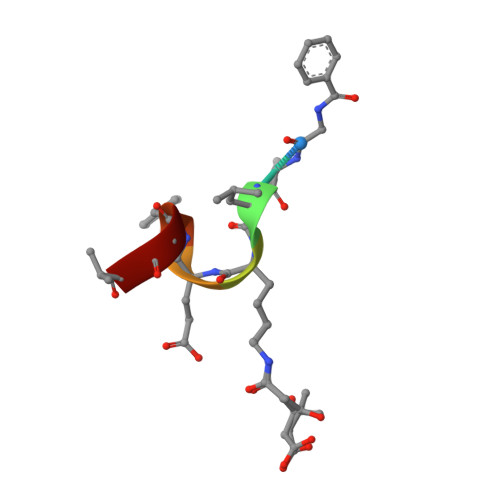
Crystal structures of the mitochondrial deacylase Sirtuin 4 reveal isoform-specific acyl recognition and regulation features.
Pannek, M., Simic, Z., Fuszard, M., Meleshin, M., Rotili, D., Mai, A., Schutkowski, M., Steegborn, C.(2017) Nat Commun 8: 1513-1513
- PubMed: 29138502
- DOI: https://doi.org/10.1038/s41467-017-01701-2
- Primary Citation of Related Structures:
5OJ7, 5OJN, 5OJO - PubMed Abstract:
Sirtuins are evolutionary conserved NAD + -dependent protein lysine deacylases. The seven human isoforms, Sirt1-7, regulate metabolism and stress responses and are considered therapeutic targets for aging-related diseases. Sirt4 locates to mitochondria and regulates fatty acid metabolism and apoptosis. In contrast to the mitochondrial deacetylase Sirt3 and desuccinylase Sirt5, no prominent deacylase activity and structural information are available for Sirt4. Here we describe acyl substrates and crystal structures for Sirt4. The enzyme shows isoform-specific acyl selectivity, with significant activity against hydroxymethylglutarylation. Crystal structures of Sirt4 from Xenopus tropicalis reveal a particular acyl binding site with an additional access channel, rationalizing its activities. The structures further identify a conserved, isoform-specific Sirt4 loop that folds into the active site to potentially regulate catalysis. Using these results, we further establish efficient Sirt4 activity assays, an unusual Sirt4 regulation by NADH, and Sirt4 effects of pharmacological modulators.
- Department of Biochemistry, University of Bayreuth, 95440, Bayreuth, Germany.
Organizational Affiliation: